当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
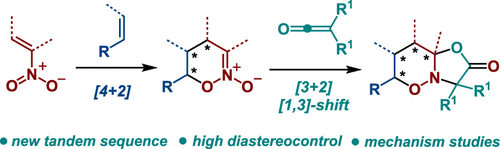
Construction of Saturated Oxazolo[3,2-b][1,2]oxazines via Tandem [3+2]-Cycloaddition/[1,3]-Rearrangement of Cyclic Nitronates and Ketenes
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-11-16 , DOI: 10.1021/acs.joc.1c01744 Roman S Malykhin 1, 2 , Ivan S Golovanov 1 , Yulia V Nelyubina 3 , Sema L Ioffe 1 , Alexey Yu Sukhorukov 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-11-16 , DOI: 10.1021/acs.joc.1c01744 Roman S Malykhin 1, 2 , Ivan S Golovanov 1 , Yulia V Nelyubina 3 , Sema L Ioffe 1 , Alexey Yu Sukhorukov 1
Affiliation
|
Reaction of six-membered cyclic nitronates with disubstituted ketenes affords hitherto unknown saturated oxazolo[3,2-b][1,2]oxazines possessing up to four contiguous stereogenic centers. The process involves a tandem of [3+2]-cycloaddition across the C═O bond of ketene, followed by a spontaneous [1,3]-rearrangement of transient vinylidene-substituted bicyclic nitrosoacetals. DFT calculations of the mechanism suggest that the [1,3]-O,C-shift proceeds through a recyclization of a biradical intermediate formed by an unusually mild homolytic cleavage of the N–O bond. The resulting products can be utilized as precursors of other fused 1,2-oxazines derivatives, in particular 1,2-oxazino-1,2,4-triazin-3-ones.
中文翻译:

通过串联[3+2]-环加成/[1,3]-环状硝酸盐和烯酮重排构建饱和恶唑并[3,2-b][1,2]恶嗪
六元环状硝酸盐与二取代乙烯酮的反应提供了迄今为止未知的饱和恶唑并[3,2- b ][1,2]恶嗪,其具有多达四个连续的立体中心。该过程涉及通过乙烯酮的 C=O 键串联 [3+2]-环加成,然后是瞬时亚乙烯基取代的双环亚硝基缩醛的自发 [1,3]-重排。该机制的 DFT 计算表明,[1,3]-O,C 位移是通过 N-O 键异常温和的均裂裂解形成的双自由基中间体的再环化进行的。所得产物可用作其他稠合1,2-恶嗪衍生物,特别是1,2-恶嗪基-1,2,4-三嗪-3-酮的前体。
更新日期:2021-12-03
中文翻译:

通过串联[3+2]-环加成/[1,3]-环状硝酸盐和烯酮重排构建饱和恶唑并[3,2-b][1,2]恶嗪
六元环状硝酸盐与二取代乙烯酮的反应提供了迄今为止未知的饱和恶唑并[3,2- b ][1,2]恶嗪,其具有多达四个连续的立体中心。该过程涉及通过乙烯酮的 C=O 键串联 [3+2]-环加成,然后是瞬时亚乙烯基取代的双环亚硝基缩醛的自发 [1,3]-重排。该机制的 DFT 计算表明,[1,3]-O,C 位移是通过 N-O 键异常温和的均裂裂解形成的双自由基中间体的再环化进行的。所得产物可用作其他稠合1,2-恶嗪衍生物,特别是1,2-恶嗪基-1,2,4-三嗪-3-酮的前体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号