当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective total syntheses of marine natural products (+)-cylindricines C, D, E and their diastereomers
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-09 , DOI: 10.1039/d1qo01408k Ying-Hong Huang 1 , Zhan-Jiang Liu 1 , Pei-Qiang Huang 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-09 , DOI: 10.1039/d1qo01408k Ying-Hong Huang 1 , Zhan-Jiang Liu 1 , Pei-Qiang Huang 1
Affiliation
|
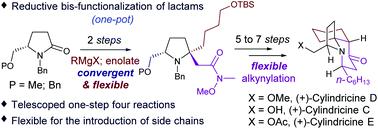
A seven-step total synthesis of (+)-cylindricine D and epi-cylindricine D is described. The efficiency relies on the highly diastereoselective reductive bis-functionalization of N,O-protected (S)-pyroglutaminol by our recently improved protocol to build the N-α-tert-alkylamine motif, and a telescoped one-step four-reaction protocol to build the fused bicyclic ring system. (+)-Cylindricine C and its 2-epimer were synthesized by essentially the same sequence in eight steps. Acetylation of the latter then delivered (+)-cylindricine E and its 2-epimer, respectively.
中文翻译:

海洋天然产物 (+)-柱状化合物 C、D、E 及其非对映体的对映选择性全合成
描述了 (+)-cylindricine D 和epi -cylindricine D 的七步全合成。效率依赖的高度非对映还原双官能N,O -保护(小号)-pyroglutaminol由我们的最近改进的协议来构建Ñ -α-叔烷基胺基序,和一个伸缩一步法四反应协议来构建稠合双环系统。(+)-Cylindricine C 和它的 2-差向异构体是按照基本相同的顺序分八步合成的。后者的乙酰化然后分别传递 (+)-cylindricine E 和它的 2-差向异构体。
更新日期:2021-12-01
中文翻译:

海洋天然产物 (+)-柱状化合物 C、D、E 及其非对映体的对映选择性全合成
描述了 (+)-cylindricine D 和epi -cylindricine D 的七步全合成。效率依赖的高度非对映还原双官能N,O -保护(小号)-pyroglutaminol由我们的最近改进的协议来构建Ñ -α-叔烷基胺基序,和一个伸缩一步法四反应协议来构建稠合双环系统。(+)-Cylindricine C 和它的 2-差向异构体是按照基本相同的顺序分八步合成的。后者的乙酰化然后分别传递 (+)-cylindricine E 和它的 2-差向异构体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号