当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanisms and origins of regioselectivities of nickel-catalyzed β,δ-vinylarylation of alkenyl esters with vinyl triflates and arylzinc reagents
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-04 , DOI: 10.1039/d1qo01153g Yupan Li 1 , Wan Xu 1 , Ting Wang 1 , Hui Chen 1 , Juan Li 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-04 , DOI: 10.1039/d1qo01153g Yupan Li 1 , Wan Xu 1 , Ting Wang 1 , Hui Chen 1 , Juan Li 1
Affiliation
|
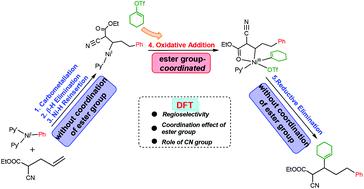
The mechanisms and origins of regioselectivity in the β,δ-vinylarylation of alkenyl esters with vinyl triflates and arylzinc reagents catalyzed by nickel catalysts were systematically explored using density functional theory calculations to focus on revealing whether the remote difunctionalization of γ,δ-alkenyl α-cyanocarboxylic esters proceeds via the coordination of an ester group. The calculations indicate that the ester group only coordinates to Ni during the oxidative addition step, which is significantly different from the commonly accepted mechanism, which involves the contraction of six-membered nickellacycles to afford five-membered nickellacycles. The origin of the regioselectivity is attributed to the β,δ-vinylarylation pathway not involving the formation of the unstable high oxidation state Ni(III) species in the phenyl migration step and encountering less steric hindrance in the oxidative addition step. The Ni–π and π–π interactions were found to account for the experimentally observed substituent-controlled reactivity.
中文翻译:

镍催化的烯基酯与乙烯基三氟甲磺酸酯和芳基锌试剂的 β,δ-乙烯基芳基化反应的机理和起源
使用密度泛函理论计算系统地探索了镍催化剂催化的烯基酯与乙烯基三氟甲磺酸酯和芳基锌试剂的 β,δ-乙烯基芳基化中区域选择性的机制和起源,重点揭示 γ,δ-烯基 α- 的远程双官能化是否存在氰基羧酸酯通过酯基的配位进行。计算表明,酯基仅在氧化加成步骤中与 Ni 配位,这与普遍接受的机制显着不同,后者涉及六元镍环收缩以提供五元镍环。区域选择性的起源归因于不涉及形成不稳定的高氧化态 Ni(III ) 苯基迁移步骤中的物质并且在氧化加成步骤中遇到较少的空间位阻。发现 Ni-π 和 π-π 相互作用解释了实验观察到的取代基控制的反应性。
更新日期:2021-11-16
中文翻译:

镍催化的烯基酯与乙烯基三氟甲磺酸酯和芳基锌试剂的 β,δ-乙烯基芳基化反应的机理和起源
使用密度泛函理论计算系统地探索了镍催化剂催化的烯基酯与乙烯基三氟甲磺酸酯和芳基锌试剂的 β,δ-乙烯基芳基化中区域选择性的机制和起源,重点揭示 γ,δ-烯基 α- 的远程双官能化是否存在氰基羧酸酯通过酯基的配位进行。计算表明,酯基仅在氧化加成步骤中与 Ni 配位,这与普遍接受的机制显着不同,后者涉及六元镍环收缩以提供五元镍环。区域选择性的起源归因于不涉及形成不稳定的高氧化态 Ni(III ) 苯基迁移步骤中的物质并且在氧化加成步骤中遇到较少的空间位阻。发现 Ni-π 和 π-π 相互作用解释了实验观察到的取代基控制的反应性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号