当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Three-component photoredox 1,2-alkylamination of styrenes with alkanes and nitrogen nucleophiles via C(sp3)–H bond cleavage
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-03 , DOI: 10.1039/d1qo01263k Jing Huang 1 , Yun-Yan Liang 1 , Xuan-Hui Ouyang 1 , Yu-Ting Xiao 1 , Jing-Hao Qin 1 , Ren-Jie Song 1 , Jin-Heng Li 1, 2, 3
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-03 , DOI: 10.1039/d1qo01263k Jing Huang 1 , Yun-Yan Liang 1 , Xuan-Hui Ouyang 1 , Yu-Ting Xiao 1 , Jing-Hao Qin 1 , Ren-Jie Song 1 , Jin-Heng Li 1, 2, 3
Affiliation
|
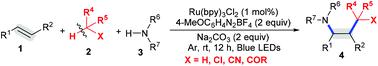
A three-component photoredox 1,2-alkylamination of styrenes involving functionalization of C(sp3)–H bonds in alkyl halides instead of functionalization of C-halogen bonds is disclosed. A variety of commercialized C(sp3)–H alkanes, including dichloromethane, acetonitrile, and acetone, used as alkyl radical precursors and solvents, were compatible with this methodology. The mechanistic study shows that alkyl radicals are generated by hydrogen atom transfer (HAT) between aryl radicals and alkyl halides.
中文翻译:

苯乙烯与烷烃和氮亲核试剂通过 C(sp3)-H 键断裂的三组分光氧化还原 1,2-烷基胺化
公开了苯乙烯的三组分光氧化还原 1,2-烷基胺化,涉及烷基卤化物中 C(sp 3 )-H 键的官能化,而不是 C-卤素键的官能化。各种商业化的 C(sp 3 )–H 烷烃,包括二氯甲烷、乙腈和丙酮,用作烷基自由基前体和溶剂,都与该方法兼容。机理研究表明,烷基自由基是通过芳基自由基和烷基卤化物之间的氢原子转移(HAT)产生的。
更新日期:2021-11-12
中文翻译:

苯乙烯与烷烃和氮亲核试剂通过 C(sp3)-H 键断裂的三组分光氧化还原 1,2-烷基胺化
公开了苯乙烯的三组分光氧化还原 1,2-烷基胺化,涉及烷基卤化物中 C(sp 3 )-H 键的官能化,而不是 C-卤素键的官能化。各种商业化的 C(sp 3 )–H 烷烃,包括二氯甲烷、乙腈和丙酮,用作烷基自由基前体和溶剂,都与该方法兼容。机理研究表明,烷基自由基是通过芳基自由基和烷基卤化物之间的氢原子转移(HAT)产生的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号