当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural and thermodynamic insights into a novel Mg2+–citrate-binding protein from the ABC transporter superfamily
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-11-11 , DOI: 10.1107/s2059798321010457 Suraj Kumar Mandal 1 , Shankar Prasad Kanaujia 1
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-11-11 , DOI: 10.1107/s2059798321010457 Suraj Kumar Mandal 1 , Shankar Prasad Kanaujia 1
Affiliation
|
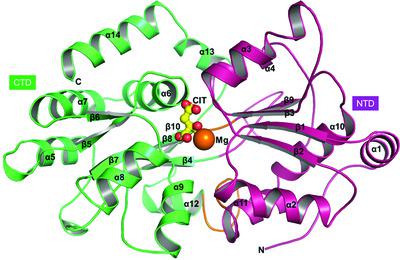
More than one third of proteins require metal ions to accomplish their functions, making them obligatory for the growth and survival of microorganisms in varying environmental niches. In prokaryotes, besides their involvement in various cellular and physiological processes, metal ions stimulate the uptake of citrate molecules. Citrate is a source of carbon and energy and is reported to be transported by secondary transporters. In Gram-positive bacteria, citrate molecules are transported in complex with divalent metal ions, whereas in Gram-negative bacteria they are translocated by Na+/citrate symporters. In this study, the presence of a novel divalent-metal-ion-complexed citrate-uptake system that belongs to the primary active ABC transporter superfamily is reported. For uptake, the metal-ion-complexed citrate molecules are sequestered by substrate-binding proteins (SBPs) and transferred to transmembrane domains for their transport. This study reports crystal structures of an Mg2+–citrate-binding protein (MctA) from the Gram-negative thermophilic bacterium Thermus thermophilus HB8 in both apo and holo forms in the resolution range 1.63–2.50 Å. Despite binding various divalent metal ions, MctA possesses the coordination geometry to bind its physiological metal ion, Mg2+. The results also suggest an extended subclassification of cluster D SBPs, which are known to bind and transport divalent-metal-ion-complexed citrate molecules. Comparative assessment of the open and closed conformations of the wild-type and mutant MctA proteins suggests a gating mechanism of ligand entry following an `asymmetric domain movement' of the N-terminal domain for substrate binding.
中文翻译:

来自 ABC 转运蛋白超家族的新型 Mg2+-柠檬酸盐结合蛋白的结构和热力学见解
超过三分之一的蛋白质需要金属离子来完成其功能,这使得它们成为微生物在不同环境生态位中生长和生存的必要条件。在原核生物中,除了参与各种细胞和生理过程外,金属离子还刺激柠檬酸盐分子的摄取。柠檬酸盐是碳和能源的来源,据报道由二级转运蛋白运输。在革兰氏阳性菌中,柠檬酸盐分子与二价金属离子复合运输,而在革兰氏阴性菌中,柠檬酸盐分子通过 Na +转运。/柠檬酸盐转运体。在这项研究中,报告了属于主要活性 ABC 转运蛋白超家族的新型二价金属离子复合柠檬酸盐吸收系统的存在。对于吸收,金属离子络合的柠檬酸盐分子被底物结合蛋白 (SBP) 隔离并转移到跨膜结构域进行转运。本研究报告了来自革兰氏阴性嗜热菌Thermus thermophilus HB8的 Mg 2+ -柠檬酸盐结合蛋白 (MctA) 的晶体结构,分辨率范围为 1.63-2.50 Å。尽管结合多种二价金属离子,MctA 具有与其生理金属离子 Mg 2+结合的配位几何结构. 结果还表明簇 D SBP 的扩展子分类,已知其结合和运输二价金属离子络合的柠檬酸盐分子。对野生型和突变型 MctA 蛋白的开放和封闭构象的比较评估表明,配体进入的门控机制是在 N 端结构域的“不对称结构域运动”后进行底物结合。
更新日期:2021-12-06
中文翻译:

来自 ABC 转运蛋白超家族的新型 Mg2+-柠檬酸盐结合蛋白的结构和热力学见解
超过三分之一的蛋白质需要金属离子来完成其功能,这使得它们成为微生物在不同环境生态位中生长和生存的必要条件。在原核生物中,除了参与各种细胞和生理过程外,金属离子还刺激柠檬酸盐分子的摄取。柠檬酸盐是碳和能源的来源,据报道由二级转运蛋白运输。在革兰氏阳性菌中,柠檬酸盐分子与二价金属离子复合运输,而在革兰氏阴性菌中,柠檬酸盐分子通过 Na +转运。/柠檬酸盐转运体。在这项研究中,报告了属于主要活性 ABC 转运蛋白超家族的新型二价金属离子复合柠檬酸盐吸收系统的存在。对于吸收,金属离子络合的柠檬酸盐分子被底物结合蛋白 (SBP) 隔离并转移到跨膜结构域进行转运。本研究报告了来自革兰氏阴性嗜热菌Thermus thermophilus HB8的 Mg 2+ -柠檬酸盐结合蛋白 (MctA) 的晶体结构,分辨率范围为 1.63-2.50 Å。尽管结合多种二价金属离子,MctA 具有与其生理金属离子 Mg 2+结合的配位几何结构. 结果还表明簇 D SBP 的扩展子分类,已知其结合和运输二价金属离子络合的柠檬酸盐分子。对野生型和突变型 MctA 蛋白的开放和封闭构象的比较评估表明,配体进入的门控机制是在 N 端结构域的“不对称结构域运动”后进行底物结合。


























 京公网安备 11010802027423号
京公网安备 11010802027423号