当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Imidodiphosphoric Acids Catalysed Asymmetric Functionaliza-tion with Thiols: Access to Oxindole Derived ɑ-Chiral Thioethers
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-11-08 , DOI: 10.1002/adsc.202100978 Xiangshuo Qin 1 , Guofeng Jiang 2 , Jigang Gao 1 , Heng Zhang 2 , Dongyang Sun 2 , guangliang zhang 1 , Liangyu Zheng 1 , Suoqin Zhang 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-11-08 , DOI: 10.1002/adsc.202100978 Xiangshuo Qin 1 , Guofeng Jiang 2 , Jigang Gao 1 , Heng Zhang 2 , Dongyang Sun 2 , guangliang zhang 1 , Liangyu Zheng 1 , Suoqin Zhang 1
Affiliation
|
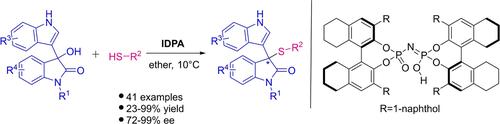
Chiral imidodiphosphoric acid-catalysed asymmetric functionalization of 3-hydroxy bisindoles was established. Sulfur-containing groups could be successfully introduced into isatin frameworks using commercially available thiols. The transformation was compatible with substrates bearing distinct substituents, both aromatic and aliphatic thiols were amenable to afford the desired products. A total of 41 reactions furnished various chiral 3-thiooxindoles in 23%–99% yield, with 72%–99% enantioselectivity.
中文翻译:

亚氨基二磷酸与硫醇催化的不对称官能化:获得羟吲哚衍生的ɑ-手性硫醚
建立了手性亚胺二磷酸催化的 3-羟基双吲哚的不对称官能化。使用市售硫醇可以成功地将含硫基团引入靛红骨架中。该转化与带有不同取代基的底物相容,芳香族和脂肪族硫醇都能够提供所需的产物。总共 41 个反应以 23%–99% 的产率提供了各种手性 3-硫代吲哚,对映选择性为 72%–99%。
更新日期:2022-01-05
中文翻译:

亚氨基二磷酸与硫醇催化的不对称官能化:获得羟吲哚衍生的ɑ-手性硫醚
建立了手性亚胺二磷酸催化的 3-羟基双吲哚的不对称官能化。使用市售硫醇可以成功地将含硫基团引入靛红骨架中。该转化与带有不同取代基的底物相容,芳香族和脂肪族硫醇都能够提供所需的产物。总共 41 个反应以 23%–99% 的产率提供了各种手性 3-硫代吲哚,对映选择性为 72%–99%。


























 京公网安备 11010802027423号
京公网安备 11010802027423号