Advanced Drug Delivery Reviews ( IF 16.1 ) Pub Date : 2021-11-08 , DOI: 10.1016/j.addr.2021.114041 Thijs Van de Vyver 1 , Stefaan C De Smedt 1 , Koen Raemdonck 1
|
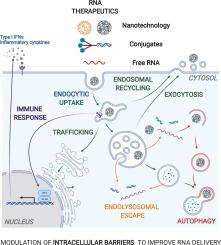
RNA therapeutics (e.g. siRNA, oligonucleotides, mRNA, etc.) show great potential for the treatment of a myriad of diseases. However, to reach their site of action in the cytosol or nucleus of target cells, multiple intra- and extracellular barriers have to be surmounted. Several non-viral delivery systems, such as nanoparticles and conjugates, have been successfully developed to meet this requirement. Unfortunately, despite these clear advances, state-of-the-art delivery agents still suffer from relatively low intracellular delivery efficiencies. Notably, our current understanding of the intracellular delivery process is largely oversimplified. Gaining mechanistic insight into how RNA formulations are processed by cells will fuel rational design of the next generation of delivery carriers. In addition, identifying which intracellular pathways contribute to productive RNA delivery could provide opportunities to boost the delivery performance of existing nanoformulations. In this review, we discuss both established as well as emerging techniques that can be used to assess the impact of different intracellular barriers on RNA transfection performance. Next, we highlight how several modulators, including small molecules but also genetic perturbation technologies, can boost RNA delivery by intervening at differing stages of the intracellular delivery process, such as cellular uptake, intracellular trafficking, endosomal escape, autophagy and exocytosis.
中文翻译:

调节细胞内途径以改善 RNA 疗法的非病毒递送
RNA 治疗剂(例如siRNA、寡核苷酸、mRNA等。) 显示出治疗多种疾病的巨大潜力。然而,为了在靶细胞的细胞质或细胞核中到达它们的作用位点,必须克服多个细胞内和细胞外屏障。已经成功开发了几种非病毒递送系统,例如纳米颗粒和缀合物,以满足这一要求。不幸的是,尽管取得了这些明显的进步,但最先进的递送剂仍然存在细胞内递送效率相对较低的问题。值得注意的是,我们目前对细胞内递送过程的理解在很大程度上过于简单化了。深入了解细胞如何处理 RNA 制剂的机制将推动下一代递送载体的合理设计。此外,确定哪些细胞内途径有助于生产性 RNA 递送,可以为提高现有纳米制剂的递送性能提供机会。在这篇综述中,我们讨论了可用于评估不同细胞内屏障对 RNA 转染性能的影响的成熟技术和新兴技术。接下来,我们将重点介绍几种调节剂,包括小分子以及遗传扰动技术,如何通过干预细胞内递送过程的不同阶段(例如细胞摄取、细胞内运输、内体逃逸、自噬和胞吐作用)来促进 RNA 递送。我们讨论了可用于评估不同细胞内屏障对 RNA 转染性能的影响的既定技术和新兴技术。接下来,我们将重点介绍几种调节剂,包括小分子以及遗传扰动技术,如何通过干预细胞内递送过程的不同阶段(例如细胞摄取、细胞内运输、内体逃逸、自噬和胞吐作用)来促进 RNA 递送。我们讨论了可用于评估不同细胞内屏障对 RNA 转染性能的影响的既定技术和新兴技术。接下来,我们将重点介绍几种调节剂,包括小分子以及遗传扰动技术,如何通过干预细胞内递送过程的不同阶段(例如细胞摄取、细胞内运输、内体逃逸、自噬和胞吐作用)来促进 RNA 递送。



























 京公网安备 11010802027423号
京公网安备 11010802027423号