当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
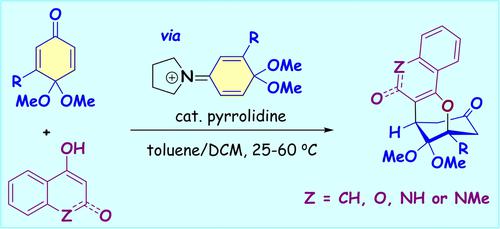
Pyrrolidine-Catalyzed Annulations of Quinone Monoacetals with Naphthols: Synthesis of 2-Oxabicyclo[3.3.1]nonane Skeletons, Transformations and Reaction Mechanism
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-11-05 , DOI: 10.1002/adsc.202101166 Xiaojie Li 1 , Xiao Tianyu 1 , Guangke He 1 , Shugao Zhu 2 , Dunru Zhu 1 , Ruwei Shen 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-11-05 , DOI: 10.1002/adsc.202101166 Xiaojie Li 1 , Xiao Tianyu 1 , Guangke He 1 , Shugao Zhu 2 , Dunru Zhu 1 , Ruwei Shen 1
Affiliation
|
Herein, we report the pyrrolidine-catalyzed annulation reaction of p-quinone monoacetals with naphthols at room temperature. The reaction is also extended to including 4-hydroxycoumarin, 4-hydroxy-1-methylcarbostyril, 4-hydroxycarbostyril, and several β-ketoesters as the nucleophiles, thereby providing a collection of bridged cyclic compounds bearing 2-oxabicyclo[3.3.1]nonane skeletons in 41–96% yields. The reaction can be adapted to gram-scale synthesis, and several transformations of the obtained bridged cyclic products are demonstrated for the preparation of polycyclic compounds that may find utility in related fields. Mechanism studies indicate the engagement of iminium intermediate in the reaction, and a bridged ring enamine intermediate can be observed by NMR.
中文翻译:

吡咯烷催化醌单缩醛与萘酚的环化:2-氧杂双环[3.3.1]壬烷骨架的合成、转化和反应机理
在此,我们报道了吡咯烷催化的对醌单缩醛与萘酚在室温下的环化反应。该反应还扩展到包括 4-羟基香豆素、4-羟基-1-甲基喹诺酮、4-羟基喹诺酮和几种 β-酮酯作为亲核试剂,从而提供一系列带有 2-氧杂双环[3.3.1]壬烷的桥接环状化合物骨骼的产率为 41-96%。该反应可以适应克级合成,并且证明了所获得的桥接环状产物的几种转化可用于制备可能在相关领域中发现实用性的多环化合物。机理研究表明,亚胺中间体参与了反应,NMR 可以观察到桥环烯胺中间体。
更新日期:2021-11-05
中文翻译:

吡咯烷催化醌单缩醛与萘酚的环化:2-氧杂双环[3.3.1]壬烷骨架的合成、转化和反应机理
在此,我们报道了吡咯烷催化的对醌单缩醛与萘酚在室温下的环化反应。该反应还扩展到包括 4-羟基香豆素、4-羟基-1-甲基喹诺酮、4-羟基喹诺酮和几种 β-酮酯作为亲核试剂,从而提供一系列带有 2-氧杂双环[3.3.1]壬烷的桥接环状化合物骨骼的产率为 41-96%。该反应可以适应克级合成,并且证明了所获得的桥接环状产物的几种转化可用于制备可能在相关领域中发现实用性的多环化合物。机理研究表明,亚胺中间体参与了反应,NMR 可以观察到桥环烯胺中间体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号