当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Dieckmann Condensation towards Spirocyclic Oxindoles Catalyzed by Amino Acid-Derived Phosphonium Salts
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-11-05 , DOI: 10.1002/adsc.202101031 Gang Zhao 1 , Longhui YU 1 , Jun Liu 1 , Hongyu Wang 2 , Lijun Xu 1 , Yu-fei Wu 3 , Changwu Zheng 4
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-11-05 , DOI: 10.1002/adsc.202101031 Gang Zhao 1 , Longhui YU 1 , Jun Liu 1 , Hongyu Wang 2 , Lijun Xu 1 , Yu-fei Wu 3 , Changwu Zheng 4
Affiliation
|
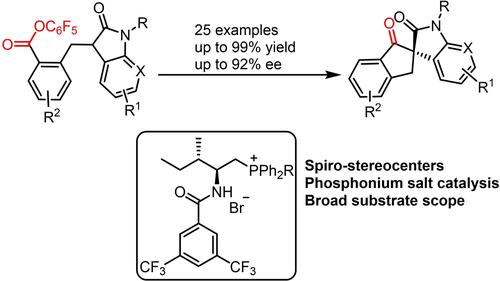
An asymmetric Dieckmann condensation towards spirocyclic oxindoles and aza-oxindoles catalyzed by amino acid-derived phosphonium salt has been developed, which expanded the applicability of asymmetric phosphonium salt catalysis in the production of 1,3-dicarbonyl compounds. This reaction is distinguished by its mild conditions (−20 °C), low catalyst loading (3–5 mol%), and broad substrate scope (25 examples). Control experiments revealed that both the catalyst‘s phosphonium skeleton and H-bonding site were required. Product transformations and a gram scale experiment were also carried out.
中文翻译:

氨基酸衍生的鏻盐催化螺环羟吲哚的不对称Dieckmann缩合反应
开发了由氨基酸衍生的鏻盐催化的螺环羟吲哚和氮杂羟吲哚的不对称Dieckmann缩合反应,扩大了不对称鏻盐催化在1,3-二羰基化合物生产中的适用性。该反应的特点在于其温和的条件(-20 °C)、低催化剂负载量(3-5 mol%)和广泛的底物范围(25 个示例)。对照实验表明,催化剂的鏻骨架和氢键位点都是必需的。还进行了产品转化和克级实验。
更新日期:2021-11-05
中文翻译:

氨基酸衍生的鏻盐催化螺环羟吲哚的不对称Dieckmann缩合反应
开发了由氨基酸衍生的鏻盐催化的螺环羟吲哚和氮杂羟吲哚的不对称Dieckmann缩合反应,扩大了不对称鏻盐催化在1,3-二羰基化合物生产中的适用性。该反应的特点在于其温和的条件(-20 °C)、低催化剂负载量(3-5 mol%)和广泛的底物范围(25 个示例)。对照实验表明,催化剂的鏻骨架和氢键位点都是必需的。还进行了产品转化和克级实验。


























 京公网安备 11010802027423号
京公网安备 11010802027423号