当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
All-d-Enantiomeric Peptide D3 Designed for Alzheimer’s Disease Treatment Dynamically Interacts with Membrane-Bound Amyloid-β Precursors
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2021-11-05 , DOI: 10.1021/acs.jmedchem.1c00632 Eduard V Bocharov 1, 2 , Lothar Gremer 1, 3, 4, 5 , Anatoly S Urban 1, 2 , Ivan S Okhrimenko 1 , Pavel E Volynsky 1, 2 , Kirill D Nadezhdin 1, 2 , Olga V Bocharova 1, 2 , Daniil A Kornilov 1 , Yuliya A Zagryadskaya 1 , Anna V Kamynina 1, 6 , Pavel K Kuzmichev 1 , Janine Kutzsche 3, 4, 5 , Najoua Bolakhrif 3, 4, 5 , Andreas Müller-Schiffmann 7 , Norbert A Dencher 1, 8 , Alexander S Arseniev 1, 2 , Roman G Efremov 1, 2, 9 , Valentin I Gordeliy 1, 3, 4, 10 , Dieter Willbold 1, 3, 4, 5
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2021-11-05 , DOI: 10.1021/acs.jmedchem.1c00632 Eduard V Bocharov 1, 2 , Lothar Gremer 1, 3, 4, 5 , Anatoly S Urban 1, 2 , Ivan S Okhrimenko 1 , Pavel E Volynsky 1, 2 , Kirill D Nadezhdin 1, 2 , Olga V Bocharova 1, 2 , Daniil A Kornilov 1 , Yuliya A Zagryadskaya 1 , Anna V Kamynina 1, 6 , Pavel K Kuzmichev 1 , Janine Kutzsche 3, 4, 5 , Najoua Bolakhrif 3, 4, 5 , Andreas Müller-Schiffmann 7 , Norbert A Dencher 1, 8 , Alexander S Arseniev 1, 2 , Roman G Efremov 1, 2, 9 , Valentin I Gordeliy 1, 3, 4, 10 , Dieter Willbold 1, 3, 4, 5
Affiliation
|
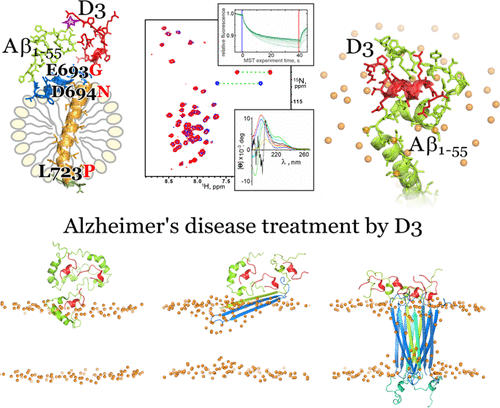
Alzheimer’s disease (AD) is a severe neurodegenerative pathology with no effective treatment known. Toxic amyloid-β peptide (Aβ) oligomers play a crucial role in AD pathogenesis. All-d-Enantiomeric peptide D3 and its derivatives were developed to disassemble and destroy cytotoxic Aβ aggregates. One of the D3-like compounds is approaching phase II clinical trials; however, high-resolution details of its disease-preventing or pharmacological actions are not completely clear. We demonstrate that peptide D3 stabilizing Aβ monomer dynamically interacts with the extracellular juxtamembrane region of a membrane-bound fragment of an amyloid precursor protein containing the Aβ sequence. MD simulations based on NMR measurement results suggest that D3 targets the amyloidogenic region, not compromising its α-helicity and preventing intermolecular hydrogen bonding, thus creating prerequisites for inhibition of early steps of Aβ conversion into β-conformation and its toxic oligomerization. An enhanced understanding of the D3 action molecular mechanism facilitates development of effective AD treatment and prevention strategies.
中文翻译:

专为阿尔茨海默病治疗而设计的全 d-对映体肽 D3 与膜结合的淀粉样蛋白-β 前体动态相互作用
阿尔茨海默病 (AD) 是一种严重的神经退行性疾病,目前尚无有效的治疗方法。有毒的淀粉样蛋白-β 肽 (Aβ) 寡聚体在 AD 发病机制中起关键作用。全部- d -对映体肽 D3 及其衍生物被开发用于分解和破坏细胞毒性 Aβ 聚集体。其中一种 D3 样化合物正在接近 II 期临床试验;然而,其预防疾病或药理作用的高分辨率细节并不完全清楚。我们证明了稳定 Aβ 单体的肽 D3 与含有 Aβ 序列的淀粉样前体蛋白的膜结合片段的细胞外近膜区域动态相互作用。基于 NMR 测量结果的 MD 模拟表明,D3 靶向淀粉样蛋白生成区域,不损害其 α-螺旋性并防止分子间氢键键合,从而为抑制 Aβ 转化为 β-构象及其毒性低聚的早期步骤创造了先决条件。
更新日期:2021-11-25
中文翻译:

专为阿尔茨海默病治疗而设计的全 d-对映体肽 D3 与膜结合的淀粉样蛋白-β 前体动态相互作用
阿尔茨海默病 (AD) 是一种严重的神经退行性疾病,目前尚无有效的治疗方法。有毒的淀粉样蛋白-β 肽 (Aβ) 寡聚体在 AD 发病机制中起关键作用。全部- d -对映体肽 D3 及其衍生物被开发用于分解和破坏细胞毒性 Aβ 聚集体。其中一种 D3 样化合物正在接近 II 期临床试验;然而,其预防疾病或药理作用的高分辨率细节并不完全清楚。我们证明了稳定 Aβ 单体的肽 D3 与含有 Aβ 序列的淀粉样前体蛋白的膜结合片段的细胞外近膜区域动态相互作用。基于 NMR 测量结果的 MD 模拟表明,D3 靶向淀粉样蛋白生成区域,不损害其 α-螺旋性并防止分子间氢键键合,从而为抑制 Aβ 转化为 β-构象及其毒性低聚的早期步骤创造了先决条件。



























 京公网安备 11010802027423号
京公网安备 11010802027423号