当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diboron-controlled product selectivity switch in copper-catalyzed decarboxylative substitutions of alkynyl cyclic carbonates
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-02 , DOI: 10.1039/d1qo01411k Guojing Pei 1 , Hui Chen 1 , Wan Xu 1 , Tao Chen 2 , Juan Li 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-11-02 , DOI: 10.1039/d1qo01411k Guojing Pei 1 , Hui Chen 1 , Wan Xu 1 , Tao Chen 2 , Juan Li 1
Affiliation
|
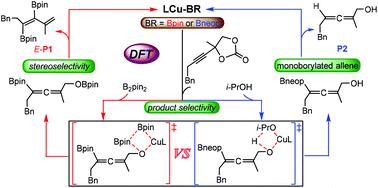
This paper presents a density functional theory study of the mechanisms and origins of diboron-controlled divergent product selectivity in the copper-catalyzed decarboxylative substitution of alkynyl cyclic carbonates. Calculation results indicate that a copper alkoxide intermediate, which is derived via the third step, i.e., CO2 extrusion, selectively determines formation of an (E)-1,2-borylated 1,3-diene product or an α-hydroxyallene product. The selected product depends on the diboron. Frontier molecular orbital analysis showed that the switchable product selectivity results from the differences among the diboron lowest unoccupied molecular orbital energies. The results show that a monoborylated allene is the precursor to the α-hydroxyallene product but not to the (E)-1,2-borylated 1,3-diene product. The stereoselectivity-determining step is Cβ-borylation. Steric hindrance is the dominant factor in the stereoselectivity.
中文翻译:

二硼控制的产物选择性转换在铜催化的炔基环状碳酸酯的脱羧取代中
本文对铜催化的炔基环状碳酸酯的脱羧取代中二硼控制的发散产物选择性的机制和起源进行了密度泛函理论研究。计算结果表明,通过第三步即CO 2挤出得到的铜醇盐中间体选择性地决定了 ( E)-1,2-硼化的 1,3-二烯产物或 α-羟基丙二烯产物。所选产品取决于二硼。前沿分子轨道分析表明,可转换产物选择性是由二硼最低未占分子轨道能量之间的差异引起的。结果表明,单硼化丙二烯是 α-羟基丙二烯产物的前体,但不是 ( E )-1,2-硼酸化 1,3-二烯产物的前体。立体选择性决定步骤是 C β -硼酸化。空间位阻是立体选择性的主要因素。
更新日期:2021-11-04
中文翻译:

二硼控制的产物选择性转换在铜催化的炔基环状碳酸酯的脱羧取代中
本文对铜催化的炔基环状碳酸酯的脱羧取代中二硼控制的发散产物选择性的机制和起源进行了密度泛函理论研究。计算结果表明,通过第三步即CO 2挤出得到的铜醇盐中间体选择性地决定了 ( E)-1,2-硼化的 1,3-二烯产物或 α-羟基丙二烯产物。所选产品取决于二硼。前沿分子轨道分析表明,可转换产物选择性是由二硼最低未占分子轨道能量之间的差异引起的。结果表明,单硼化丙二烯是 α-羟基丙二烯产物的前体,但不是 ( E )-1,2-硼酸化 1,3-二烯产物的前体。立体选择性决定步骤是 C β -硼酸化。空间位阻是立体选择性的主要因素。


























 京公网安备 11010802027423号
京公网安备 11010802027423号