当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ambiguities in and completeness of SAS data analysis of membrane proteins: the case of the sensory rhodopsin II–transducer complex
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-11-02 , DOI: 10.1107/s2059798321009542 Yury L Ryzhykau 1 , Alexey V Vlasov 1 , Philipp S Orekhov 1 , Maksim I Rulev 2 , Andrey V Rogachev 1 , Anastasia D Vlasova 1 , Alexander S Kazantsev 1 , Dmitry P Verteletskiy 1 , Vadim V Skoi 1 , Martha E Brennich 3 , Petra Pernot 2 , Tatiana N Murugova 1 , Valentin I Gordeliy 1 , Alexander I Kuklin 1
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-11-02 , DOI: 10.1107/s2059798321009542 Yury L Ryzhykau 1 , Alexey V Vlasov 1 , Philipp S Orekhov 1 , Maksim I Rulev 2 , Andrey V Rogachev 1 , Anastasia D Vlasova 1 , Alexander S Kazantsev 1 , Dmitry P Verteletskiy 1 , Vadim V Skoi 1 , Martha E Brennich 3 , Petra Pernot 2 , Tatiana N Murugova 1 , Valentin I Gordeliy 1 , Alexander I Kuklin 1
Affiliation
|
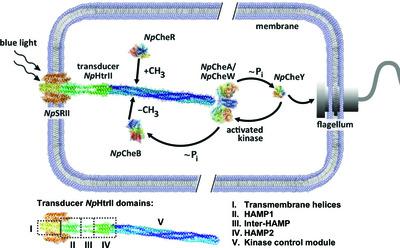
Membrane proteins (MPs) play vital roles in the function of cells and are also major drug targets. Structural information on proteins is vital for understanding their mechanism of function and is critical for the development of drugs. However, obtaining high-resolution structures of membrane proteins, in particular, under native conditions is still a great challenge. In such cases, the low-resolution methods small-angle X-ray and neutron scattering (SAXS and SANS) might provide valuable structural information. However, in some cases small-angle scattering (SAS) provides ambiguous ab initio structural information if complementary measurements are not performed and/or a priori information on the protein is not taken into account. Understanding the nature of the limitations may help to overcome these problems. One of the main problems of SAS data analysis of solubilized membrane proteins is the contribution of the detergent belt surrounding the MP. Here, a comprehensive analysis of how the detergent belt contributes to the SAS data of a membrane-protein complex of sensory rhodopsin II with its cognate transducer from Natronomonas pharaonis (NpSRII–NpHtrII) was performed. The influence of the polydispersity of NpSRII–NpHtrII oligomerization is the second problem that is addressed here. It is shown that inhomogeneity in the scattering length density of the detergent belt surrounding a membrane part of the complex and oligomerization polydispersity significantly impacts on SAXS and SANS profiles, and therefore on 3D ab initio structures. It is described how both problems can be taken into account to improve the quality of SAS data treatment. Since SAS data for MPs are usually obtained from solubilized proteins, and their detergent belt and, to a certain extent, oligomerization polydispersity are sufficiently common phenomena, the approaches proposed in this work might be used in SAS studies of different MPs.
中文翻译:

膜蛋白 SAS 数据分析的模糊性和完整性:以感觉视紫红质 II-传感器复合物为例
膜蛋白(MPs)在细胞功能中起着至关重要的作用,也是主要的药物靶点。蛋白质的结构信息对于了解其功能机制至关重要,对于药物的开发也至关重要。然而,获得膜蛋白的高分辨率结构,特别是在天然条件下,仍然是一个巨大的挑战。在这种情况下,低分辨率方法小角度 X 射线和中子散射(SAXS 和 SANS)可能会提供有价值的结构信息。然而,在某些情况下,如果不执行补充测量和/或先验,小角散射 (SAS) 会提供模糊的从头算结构信息不考虑蛋白质的信息。了解限制的性质可能有助于克服这些问题。溶解膜蛋白的 SAS 数据分析的主要问题之一是 MP 周围的洗涤剂带的贡献。在这里,对洗涤剂带如何对感觉视紫红质 II 的膜蛋白复合物及其来自Natronomonas pharaonis ( Np SRII– Np HtrII) 的同源传感器的 SAS 数据进行了全面分析。Np SRII– Np多分散性的影响HtrII 寡聚化是这里要解决的第二个问题。结果表明,围绕复合物的膜部分的洗涤剂带的散射长度密度的不均匀性和低聚多分散性显着影响 SAXS 和 SANS 分布,因此影响 3D从头算结构。描述了如何考虑这两个问题以提高 SAS 数据处理的质量。由于 MPs 的 SAS 数据通常是从溶解的蛋白质中获得的,它们的去污剂带和在一定程度上,低聚多分散性是足够普遍的现象,因此本工作中提出的方法可能用于不同 MPs 的 SAS 研究。
更新日期:2021-11-03
中文翻译:

膜蛋白 SAS 数据分析的模糊性和完整性:以感觉视紫红质 II-传感器复合物为例
膜蛋白(MPs)在细胞功能中起着至关重要的作用,也是主要的药物靶点。蛋白质的结构信息对于了解其功能机制至关重要,对于药物的开发也至关重要。然而,获得膜蛋白的高分辨率结构,特别是在天然条件下,仍然是一个巨大的挑战。在这种情况下,低分辨率方法小角度 X 射线和中子散射(SAXS 和 SANS)可能会提供有价值的结构信息。然而,在某些情况下,如果不执行补充测量和/或先验,小角散射 (SAS) 会提供模糊的从头算结构信息不考虑蛋白质的信息。了解限制的性质可能有助于克服这些问题。溶解膜蛋白的 SAS 数据分析的主要问题之一是 MP 周围的洗涤剂带的贡献。在这里,对洗涤剂带如何对感觉视紫红质 II 的膜蛋白复合物及其来自Natronomonas pharaonis ( Np SRII– Np HtrII) 的同源传感器的 SAS 数据进行了全面分析。Np SRII– Np多分散性的影响HtrII 寡聚化是这里要解决的第二个问题。结果表明,围绕复合物的膜部分的洗涤剂带的散射长度密度的不均匀性和低聚多分散性显着影响 SAXS 和 SANS 分布,因此影响 3D从头算结构。描述了如何考虑这两个问题以提高 SAS 数据处理的质量。由于 MPs 的 SAS 数据通常是从溶解的蛋白质中获得的,它们的去污剂带和在一定程度上,低聚多分散性是足够普遍的现象,因此本工作中提出的方法可能用于不同 MPs 的 SAS 研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号