Journal of Catalysis ( IF 7.3 ) Pub Date : 2021-10-27 , DOI: 10.1016/j.jcat.2021.10.027 Dingkai Chen 1 , Dimitrios K. Niakolas 2 , Vasiliki Papaefthimiou 1 , Evangelia Ioannidou 2 , Stylianos G. Neophytides 2 , Spyridon Zafeiratos 1
|
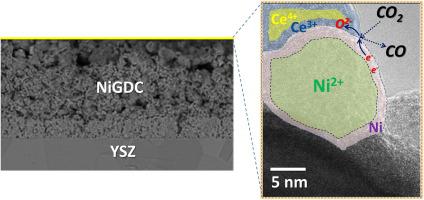
The effect of the surface state on the electrochemical performance of nickel gadolinium-doped ceria (NiGDC) cermet electrodes in direct CO2 electrolysis was studied by operando near ambient pressure X-ray photoelectron spectroscopy combined with on-line gas phase and electrical measurements. The CO2 electrolysis was limited at overpotentials below the carbon deposition threshold to avoid irreversible cathode deactivation. The results revealed the dynamic evolution of the NiGDC electrode surface and disclose the side reactions associated to electrode activation in CO2 electrolysis. Comparison of reduced and oxidized electrodes shows that metallic Ni is a prerequisite for CO2 electrolysis, at least at low potentials, suggesting that CO2 electoreduction occurs primarily at the three phase boundaries between gas, metallic nickel and partially reduced ceria. We also provide evidences of in situ reduction of NiO upon polarization in CO2, implying that addition of reductive gases to CO2 is not indispensable to maintain the cermet electrode in the reduced state. Inspired by this observation, we use a conventional button cell setup to demonstrate improved i-V characteristics of NiGDC electrodes in direct CO2 electrolysis as compared to CO2/H2 fuel conditions and we rationalize this behavior based on NAP-XPS results.
中文翻译:

镍/钆掺杂氧化铈阴极的表面状态如何影响直接 CO2 电解中的电化学性能
表面状态的镍钆掺杂的二氧化铈(NiGDC)金属陶瓷电极的直接CO的电化学性能的影响2通过研究电解operando接近环境压力X射线光电子能谱法与组合的在线气相和电测量。CO 2电解被限制在低于碳沉积阈值的过电位以避免不可逆的阴极失活。结果揭示了NiGDC电极表面的动态演化,并揭示了与CO 2电解中电极活化相关的副反应。还原和氧化电极的比较表明金属 Ni 是 CO 2的先决条件电解,至少在低电位下,表明 CO 2 电还原主要发生在气体、金属镍和部分还原的二氧化铈之间的三相边界处。我们还提供了在CO 2 中极化时 NiO原位还原的证据,这意味着向 CO 2中添加还原性气体对于将金属陶瓷电极保持在还原状态并不是必不可少的。受此观察的启发,我们使用传统的纽扣电池装置来证明与 CO 2 /H 2相比,NiGDC 电极在直接 CO 2电解中的i- V 特性得到改善 燃料条件,我们根据 NAP-XPS 结果合理化这种行为。


























 京公网安备 11010802027423号
京公网安备 11010802027423号