当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dual roles of bisphosphines and epoxides: Rh-catalyzed highly chemoselective and diastereoselective (3 + 2) transannulations of 1,2,3-thiadiazoles with cyanoepoxides
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-10-18 , DOI: 10.1039/d1qo01220g Ziyang Dong 1 , Cunzhi Chen 1 , Jing Wang 1 , Jiaxi Xu 1 , Zhanhui Yang 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-10-18 , DOI: 10.1039/d1qo01220g Ziyang Dong 1 , Cunzhi Chen 1 , Jing Wang 1 , Jiaxi Xu 1 , Zhanhui Yang 1
Affiliation
|
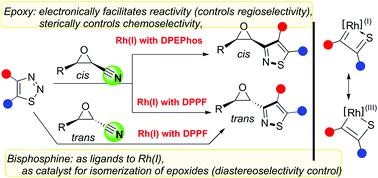
A highly diastereoselective and chemoselective (3 + 2) transannulation of 1,2,3-thiadiazoles with cyanoepoxides has been discovered. The use of sterically larger DPEPhos allows the preparation of cis-epoxyl isothiazoles from cis-cyanoepoxides in up to 95% yields and 100 : 0 dr, while the use of sterically smaller DPPF allows the synthesis of trans-products from cis- or trans-cyanoepoxides in up to 95% yields and 100 : 0 dr. Bisphosphines and epoxides play dual roles. Bisphosphines serve not only as ligands, but also as catalysts to catalyze the isomerization of cis-epoxides to trans-isomers. The diastereoselectivity is controlled by the kinetic competition between the direct transannulation of cis-cyanoepoxides and the bisphosphine-catalyzed isomerization of cis-products. The epoxy group's large steric hindrance guarantees excellent chemoselectivity toward the (3 + 2) annulation, and its electron-withdrawing ability significantly improves the reactivity of the adjacent cyano group. To address the controversy over the organorhodium intermediates, we suggest the resonation between Lee's umpolung 1,3-dipoles, Bao's cyclometalated Rh(III) complexes, and our thioacyl-coordinated Rh(I) carbenes. Stereospecific access to isothiazole-fused γ-lactone is also developed.
中文翻译:

双膦和环氧化物的双重作用:Rh 催化的 1,2,3-噻二唑与氰基环氧化物的高度化学选择性和非对映选择性 (3 + 2) 环化
已发现 1,2,3-噻二唑与氰基环氧化物的高度非对映选择性和化学选择性 (3 + 2) 环化。使用空间较大DPEPhos允许的制备顺式-epoxyl从异噻唑的顺式中-cyanoepoxides高达95倍%的产率和100:0博士,而使用空间较小DPPF允许的合成反式-products从顺式-或反式-氰基环氧化物的产率高达 95% 和 100 : 0 dr。双膦和环氧化物具有双重作用。双膦不仅作为配体,而且作为催化剂催化顺式环氧化物异构化为反式-异构体。非对映选择性由顺式氰基环氧化物的直接环化和双膦催化的顺式产物异构化之间的动力学竞争控制。环氧基团的大空间位阻保证了对(3+2)环化具有优异的化学选择性,其吸电子能力显着提高了相邻氰基的反应性。为了解决关于有机铑中间体的争议,我们建议 Lee 的 umpolung 1,3-偶极子、Bao 的环金属化 Rh( III ) 配合物和我们的硫酰基配位的 Rh( I ) 卡宾之间的共振。还开发了对异噻唑稠合 γ-内酯的立体特异性访问。
更新日期:2021-10-27
中文翻译:

双膦和环氧化物的双重作用:Rh 催化的 1,2,3-噻二唑与氰基环氧化物的高度化学选择性和非对映选择性 (3 + 2) 环化
已发现 1,2,3-噻二唑与氰基环氧化物的高度非对映选择性和化学选择性 (3 + 2) 环化。使用空间较大DPEPhos允许的制备顺式-epoxyl从异噻唑的顺式中-cyanoepoxides高达95倍%的产率和100:0博士,而使用空间较小DPPF允许的合成反式-products从顺式-或反式-氰基环氧化物的产率高达 95% 和 100 : 0 dr。双膦和环氧化物具有双重作用。双膦不仅作为配体,而且作为催化剂催化顺式环氧化物异构化为反式-异构体。非对映选择性由顺式氰基环氧化物的直接环化和双膦催化的顺式产物异构化之间的动力学竞争控制。环氧基团的大空间位阻保证了对(3+2)环化具有优异的化学选择性,其吸电子能力显着提高了相邻氰基的反应性。为了解决关于有机铑中间体的争议,我们建议 Lee 的 umpolung 1,3-偶极子、Bao 的环金属化 Rh( III ) 配合物和我们的硫酰基配位的 Rh( I ) 卡宾之间的共振。还开发了对异噻唑稠合 γ-内酯的立体特异性访问。


























 京公网安备 11010802027423号
京公网安备 11010802027423号