当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Integrated Point-of-Care Molecular Diagnostic Devices for Infectious Diseases
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2021-10-26 , DOI: 10.1021/acs.accounts.1c00385 Wenpeng Liu 1 , Fei Yue 1 , Luke P Lee 1, 2, 3
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2021-10-26 , DOI: 10.1021/acs.accounts.1c00385 Wenpeng Liu 1 , Fei Yue 1 , Luke P Lee 1, 2, 3
Affiliation
|
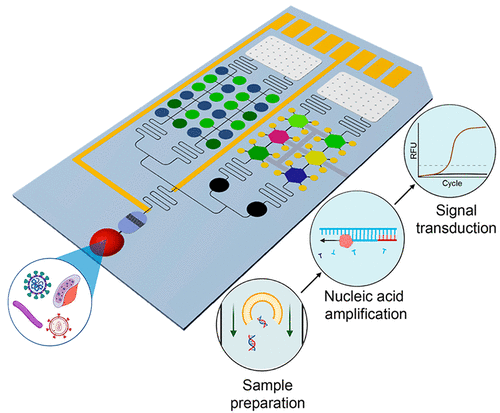
The global outbreaks of deadly infectious diseases caused by pathogenic microorganisms have threatened public health worldwide and significantly motivated scientists to satisfy an urgent need for a rapid and accurate detection of pathogens. Traditionally, the culture-based technique is considered as the gold standard for pathogen detection, yet it has a long turnaround time due to the overnight culturing and pathogen isolation. Alternatively, nucleic acid amplification tests provide a relatively shorter turnaround time to identify whether pathogens exist in individuals with high sensitivity and high specificity. In most cases, nucleic acid amplification tests undergo three steps: sample preparation, nucleic acid amplification, and signal transduction. Despite the explosive advancement in nucleic acid amplification and signal transduction technologies, the complex and labor-intensive sample preparation steps remain a bottleneck to create a transformative integrated point-of-care (POC) molecular diagnostic device. Researchers have attempted to simplify and integrate the sample preparations for nucleic acid-based molecular diagnostic devices with innovative progress in integration strategies, engineered materials, reagent storages, and fluid actuation. Therefore, understanding the know-how and obtaining truthful knowledge of existing integrated POC molecular diagnostic devices comprising sample preparations, nucleic acid amplification, and signal transduction can generate innovative solutions to achieve personalized precision medicine and improve global health.
中文翻译:

用于传染病的综合即时分子诊断设备
由病原微生物引起的致命传染病的全球爆发已威胁到全世界的公共健康,极大地激发了科学家们对快速准确检测病原体的迫切需求。传统上,基于培养的技术被认为是病原体检测的金标准,但由于过夜培养和病原体分离,它的周转时间很长。或者,核酸扩增测试提供相对较短的周转时间,以高灵敏度和高特异性识别个体中是否存在病原体。在大多数情况下,核酸扩增测试经历三个步骤:样品制备、核酸扩增和信号转导。尽管核酸扩增和信号转导技术取得了爆炸性进展,但复杂且劳动密集型的样品制备步骤仍然是创建变革性集成即时 (POC) 分子诊断设备的瓶颈。研究人员试图通过在集成策略、工程材料、试剂储存和流体驱动方面的创新进展来简化和集成基于核酸的分子诊断设备的样品制备。因此,了解现有的包括样本制备、核酸扩增和信号转导在内的集成POC分子诊断设备的技术诀窍并获得真实知识,可以产生创新的解决方案,以实现个性化精准医疗,改善全球健康。复杂且劳动密集型的样品制备步骤仍然是创建变革性集成即时 (POC) 分子诊断设备的瓶颈。研究人员试图通过在集成策略、工程材料、试剂储存和流体驱动方面的创新进展来简化和集成基于核酸的分子诊断设备的样品制备。因此,了解现有的包括样本制备、核酸扩增和信号转导在内的集成POC分子诊断设备的技术诀窍并获得真实知识,可以产生创新的解决方案,以实现个性化精准医疗,改善全球健康。复杂且劳动密集型的样品制备步骤仍然是创建变革性集成即时 (POC) 分子诊断设备的瓶颈。研究人员试图通过在集成策略、工程材料、试剂储存和流体驱动方面的创新进展来简化和集成基于核酸的分子诊断设备的样品制备。因此,了解现有的包括样本制备、核酸扩增和信号转导在内的集成POC分子诊断设备的技术诀窍并获得真实知识,可以产生创新的解决方案,以实现个性化精准医疗,改善全球健康。研究人员试图通过在集成策略、工程材料、试剂储存和流体驱动方面的创新进展来简化和集成基于核酸的分子诊断设备的样品制备。因此,了解现有的包括样本制备、核酸扩增和信号转导在内的集成POC分子诊断设备的技术诀窍并获得真实知识,可以产生创新的解决方案,以实现个性化精准医疗,改善全球健康。研究人员试图通过在集成策略、工程材料、试剂储存和流体驱动方面的创新进展来简化和集成基于核酸的分子诊断设备的样品制备。因此,了解现有的包括样本制备、核酸扩增和信号转导在内的集成POC分子诊断设备的专有技术并获得真实知识,可以产生创新解决方案,实现个性化精准医疗,改善全球健康。
更新日期:2021-11-16
中文翻译:

用于传染病的综合即时分子诊断设备
由病原微生物引起的致命传染病的全球爆发已威胁到全世界的公共健康,极大地激发了科学家们对快速准确检测病原体的迫切需求。传统上,基于培养的技术被认为是病原体检测的金标准,但由于过夜培养和病原体分离,它的周转时间很长。或者,核酸扩增测试提供相对较短的周转时间,以高灵敏度和高特异性识别个体中是否存在病原体。在大多数情况下,核酸扩增测试经历三个步骤:样品制备、核酸扩增和信号转导。尽管核酸扩增和信号转导技术取得了爆炸性进展,但复杂且劳动密集型的样品制备步骤仍然是创建变革性集成即时 (POC) 分子诊断设备的瓶颈。研究人员试图通过在集成策略、工程材料、试剂储存和流体驱动方面的创新进展来简化和集成基于核酸的分子诊断设备的样品制备。因此,了解现有的包括样本制备、核酸扩增和信号转导在内的集成POC分子诊断设备的技术诀窍并获得真实知识,可以产生创新的解决方案,以实现个性化精准医疗,改善全球健康。复杂且劳动密集型的样品制备步骤仍然是创建变革性集成即时 (POC) 分子诊断设备的瓶颈。研究人员试图通过在集成策略、工程材料、试剂储存和流体驱动方面的创新进展来简化和集成基于核酸的分子诊断设备的样品制备。因此,了解现有的包括样本制备、核酸扩增和信号转导在内的集成POC分子诊断设备的技术诀窍并获得真实知识,可以产生创新的解决方案,以实现个性化精准医疗,改善全球健康。复杂且劳动密集型的样品制备步骤仍然是创建变革性集成即时 (POC) 分子诊断设备的瓶颈。研究人员试图通过在集成策略、工程材料、试剂储存和流体驱动方面的创新进展来简化和集成基于核酸的分子诊断设备的样品制备。因此,了解现有的包括样本制备、核酸扩增和信号转导在内的集成POC分子诊断设备的技术诀窍并获得真实知识,可以产生创新的解决方案,以实现个性化精准医疗,改善全球健康。研究人员试图通过在集成策略、工程材料、试剂储存和流体驱动方面的创新进展来简化和集成基于核酸的分子诊断设备的样品制备。因此,了解现有的包括样本制备、核酸扩增和信号转导在内的集成POC分子诊断设备的技术诀窍并获得真实知识,可以产生创新的解决方案,以实现个性化精准医疗,改善全球健康。研究人员试图通过在集成策略、工程材料、试剂储存和流体驱动方面的创新进展来简化和集成基于核酸的分子诊断设备的样品制备。因此,了解现有的包括样本制备、核酸扩增和信号转导在内的集成POC分子诊断设备的专有技术并获得真实知识,可以产生创新解决方案,实现个性化精准医疗,改善全球健康。



























 京公网安备 11010802027423号
京公网安备 11010802027423号