Water Research ( IF 12.8 ) Pub Date : 2021-10-25 , DOI: 10.1016/j.watres.2021.117795 Yan Wang 1 , Huiyu Dong 1 , Wenlei Qin 1 , Jin Li 2 , Zhimin Qiang 1
|
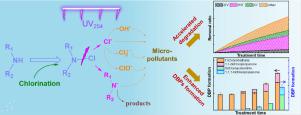
Due to the wide-presence of organic amines in natural waters, organic chloramines are commonly formed during (pre-)chlorination. With the increasing application of UV disinfection in water treatment, both the activation mechanism of organic chloramine by UV photolysis and its subsequent impact on water quality are not clear. Using sarcosine (Sar) as an amine group-containing compound, it was found that organic chloramines (i.e., Cl-Sar) would be firstly formed during chlorination even in the presence of natural organic matter. Compared with self-decay of Cl-Sar, UV photolysis accelerated Cl-Sar decomposition and induced N Cl bond cleavage. Using metoprolol (MTP) as a model micro-pollutant, UV-activated Cl-Sar (UV/Cl-Sar) can accelerate micro-pollutant degradation, attributed to reactive radicals formation. HO• and Cl• were important contributors, with a total contribution of 45%‒64%. Moreover, the degradation rate of MTP by UV/Cl-Sar was pH-dependent, which monotonically increased from 0.044 to 0.065 min‒1 under pHs 5.5‒8.5. Although the activation of organic chloramine by UV could accelerate micro-pollutant degradation, UV/Cl-Sar treatment could also enhance disinfection by-products formation. Trichloromethane (TCM) formation was observed during MTP degradation by UV/Cl-Sar. After post-chlorination, TCM, 1,1-dichloropropanone, 1,1,1-trichloropropanone, and dichloroacetonitrile were detected. Their individual and total concentrations were all positively proportional to UV/Cl-Sar treatment time. The total concentration with 30 min treatment (66.93 μg L‒1) was about 2.3 times that with 1 min treatment (28.76 μg L‒1). Finally, the accelerated effect was verified with Cl-glycine and Cl-alanine. It is expected to unravel the non-negligible role of organic chloramine on water quality during UV disinfection.
Cl bond cleavage. Using metoprolol (MTP) as a model micro-pollutant, UV-activated Cl-Sar (UV/Cl-Sar) can accelerate micro-pollutant degradation, attributed to reactive radicals formation. HO• and Cl• were important contributors, with a total contribution of 45%‒64%. Moreover, the degradation rate of MTP by UV/Cl-Sar was pH-dependent, which monotonically increased from 0.044 to 0.065 min‒1 under pHs 5.5‒8.5. Although the activation of organic chloramine by UV could accelerate micro-pollutant degradation, UV/Cl-Sar treatment could also enhance disinfection by-products formation. Trichloromethane (TCM) formation was observed during MTP degradation by UV/Cl-Sar. After post-chlorination, TCM, 1,1-dichloropropanone, 1,1,1-trichloropropanone, and dichloroacetonitrile were detected. Their individual and total concentrations were all positively proportional to UV/Cl-Sar treatment time. The total concentration with 30 min treatment (66.93 μg L‒1) was about 2.3 times that with 1 min treatment (28.76 μg L‒1). Finally, the accelerated effect was verified with Cl-glycine and Cl-alanine. It is expected to unravel the non-negligible role of organic chloramine on water quality during UV disinfection.
中文翻译:

通过紫外线光解活化有机氯胺:一种不可忽略的氧化剂,用于微量污染物减排和消毒副产物形成
由于天然水中广泛存在有机胺,因此在(预)氯化过程中通常会形成有机氯胺。随着紫外线消毒在水处理中的应用越来越广泛,紫外线光解对有机氯胺的活化机制及其对水质的后续影响均不清楚。使用肌氨酸(Sar)作为含胺基的化合物,发现即使在天然有机物存在的情况下,氯化过程中也会首先形成有机氯胺(即Cl-Sar)。与 Cl-Sar 的自衰变相比,UV 光解加速了 Cl-Sar 的分解并诱导了 N  Cl 键的断裂。使用美托洛尔 (MTP) 作为模型微污染物,紫外线激活的 Cl-Sar (UV/Cl-Sar) 可以加速微污染物降解,这归因于反应性自由基的形成。何•和Cl •是重要的贡献者,总贡献率为45%~64%。此外,UV/Cl-Sar 对 MTP 的降解速率具有 pH 依赖性,在 pH 5.5-8.5 下从 0.044 单调增加到 0.065 min -1。尽管紫外线对有机氯胺的活化可以加速微污染物的降解,但紫外线/Cl-Sar 处理也可以增强消毒副产物的形成。在通过 UV/Cl-Sar 降解 MTP 期间观察到三氯甲烷 (TCM) 的形成。后氯化后,检测到TCM、1,1-二氯丙酮、1,1,1-三氯丙酮和二氯乙腈。它们的个体和总浓度都与 UV/Cl-Sar 处理时间成正比。处理 30 分钟后的总浓度(66.93 μg L ‒1) 是处理 1 分钟 (28.76 μg L ‒1 ) 的约 2.3 倍。最后,用 Cl-甘氨酸和 Cl-丙氨酸验证了加速效果。有望在紫外线消毒过程中阐明有机氯胺对水质的不可忽视的作用。
Cl 键的断裂。使用美托洛尔 (MTP) 作为模型微污染物,紫外线激活的 Cl-Sar (UV/Cl-Sar) 可以加速微污染物降解,这归因于反应性自由基的形成。何•和Cl •是重要的贡献者,总贡献率为45%~64%。此外,UV/Cl-Sar 对 MTP 的降解速率具有 pH 依赖性,在 pH 5.5-8.5 下从 0.044 单调增加到 0.065 min -1。尽管紫外线对有机氯胺的活化可以加速微污染物的降解,但紫外线/Cl-Sar 处理也可以增强消毒副产物的形成。在通过 UV/Cl-Sar 降解 MTP 期间观察到三氯甲烷 (TCM) 的形成。后氯化后,检测到TCM、1,1-二氯丙酮、1,1,1-三氯丙酮和二氯乙腈。它们的个体和总浓度都与 UV/Cl-Sar 处理时间成正比。处理 30 分钟后的总浓度(66.93 μg L ‒1) 是处理 1 分钟 (28.76 μg L ‒1 ) 的约 2.3 倍。最后,用 Cl-甘氨酸和 Cl-丙氨酸验证了加速效果。有望在紫外线消毒过程中阐明有机氯胺对水质的不可忽视的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号