The Journal of Supercritical Fluids ( IF 3.9 ) Pub Date : 2021-10-20 , DOI: 10.1016/j.supflu.2021.105454 Dadan Ramdan 1 , Mohsen Najmi 2 , Halimeh Rajabzadeh 3 , Marischa Elveny 4 , Seyed Mehdi Seyed Alizadeh 5 , Reza Shahriari 6
|
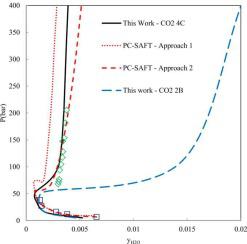
The aim of this work is to provide a reliable model to predict CO2 solubility in formation brines using an equation of state (EoS). In this regard, the electrolyte perturbed hard sphere chain equation of state (e-PHSC) was utilized to describe the solubility of carbon dioxide in aqueous electrolyte solutions. Using the ion-specific parameters and a binary interaction parameter between ions and CO2, CO2 solubilities in single electrolyte solutions (NaCl, KCl, CaCl2, MgCl2, Na2SO4, and K2SO4) were calculated. Then the model capability was evaluated for CO2 solubility in mixed-electrolyte solutions. Obtained results prove that e-PHSC EoS can be used for the prediction of CO2 solubility in mixed electrolyte solutions without any additional adjustable parameters. Finally, the predictive capacity of the e-PHSC EoS was explored for two synthetic formation brines up to 423 K. The result shows that the CO2 solubility in formation brines can be efficiently predicted using e-PHSC EoS.
中文翻译:

使用 e-PHSC 状态方程预测 CO2 在电解质溶液中的溶解度
这项工作的目的是提供一个可靠的模型来使用状态方程 (EoS)预测地层盐水中的CO 2溶解度。在这方面,电解质扰动硬球链状态方程(e-PHSC)被用来描述二氧化碳在电解质水溶液中的溶解度。使用离子特定参数和离子与 CO 2之间的二元相互作用参数,计算了单一电解质溶液(NaCl、KCl、CaCl 2、MgCl 2、Na 2 SO 4和 K 2 SO 4)中的CO 2溶解度。然后针对 CO 2评估模型能力在混合电解质溶液中的溶解度。获得的结果证明,e-PHSC EoS 可用于预测混合电解质溶液中的 CO 2溶解度,而无需任何额外的可调参数。最后,探讨了 e-PHSC EoS 对两种高达 423 K 的合成地层盐水的预测能力。结果表明,使用 e-PHSC EoS 可以有效地预测地层盐水中的 CO 2溶解度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号