Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2021-10-20 , DOI: 10.1016/j.jhazmat.2021.127548 Zijun Peng 1 , Qiangzhen Yang 1 , Ranna Yeerken 1 , Jun Chen 1 , Xurui Liu 1 , Xinhong Li 1
|
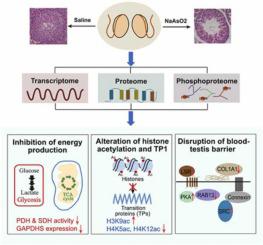
Arsenic (As), a widespread environmental contaminant, can induce serious male reproductive injury; however, the underlying mechanisms remain unclear. Multi-omics analyses, including transcriptome, proteome, and phosphoproteome could promote our understanding of As-induced male reproductive toxicity. Here, we established the reproductive injured mice model by intraperitoneal injection of NaAsO2 (8 mg/kg body weight), which was validated by reduced reproductive cells, sperm motility, and litter size. The followed multi-omics analyses of mice revealed that As exposure inhibited ATP production by decreasing the expression of proteins HK1, and GAPDHS, and the enzymatic activities of PDH and SDH. The inhibition of mitochondrial activity and increase in HDAC2 and MTA3 dysregulated the lysine acetylation levels of histone and global proteins. Specifically, the downregulated histones H4K5ac and H4K12ac and upregulated histone H3K9ac disordered the distribution of TP1 to interfere with spermatogenesis. Moreover, As could reduce the expression of COL1A1, RAB13, and LSR to disrupt the junctions between seminiferous tubules, and thereinto, the inhibition of RAB13 increased PKA-dependent phosphorylation. Our study reveals that As causes male reproductive toxicity through decreasing energy production, altering histone acetylation, and impairing cell junctions. Our findings provide basic data for further studies on As reproductive toxicity.
中文翻译:

多组学分析揭示了砷诱导小鼠雄性生殖毒性的机制
砷 (As) 是一种广泛存在的环境污染物,可导致严重的男性生殖损伤;然而,潜在的机制仍不清楚。多组学分析,包括转录组、蛋白质组和磷酸化蛋白质组,可以促进我们对 As 诱导的雄性生殖毒性的理解。在这里,我们通过腹膜内注射 NaAsO2(8 mg/kg 体重)建立了生殖损伤小鼠模型,并通过减少生殖细胞、精子活力和产仔数进行了验证。随后对小鼠的多组学分析表明,As 暴露通过降低蛋白质 HK1 和 GAPDHS 的表达以及 PDH 和 SDH 的酶活性来抑制 ATP 的产生。线粒体活性的抑制和 HDAC2 和 MTA3 的增加使组蛋白和全局蛋白的赖氨酸乙酰化水平失调。具体来说,下调的组蛋白 H4K5ac 和 H4K12ac 和上调的组蛋白 H3K9ac 扰乱了 TP1 的分布以干扰精子发生。此外,As 可以降低 COL1A1、RAB13 和 LSR 的表达,从而破坏曲细精管之间的连接,其中,RAB13 的抑制增加了 PKA 依赖性磷酸化。我们的研究表明,As 通过降低能量产生、改变组蛋白乙酰化和损害细胞连接而导致男性生殖毒性。我们的研究结果为进一步研究砷的生殖毒性提供了基础数据。其中,RAB13的抑制增加了PKA依赖性磷酸化。我们的研究表明,As 通过降低能量产生、改变组蛋白乙酰化和损害细胞连接而导致男性生殖毒性。我们的研究结果为进一步研究砷的生殖毒性提供了基础数据。其中,RAB13的抑制增加了PKA依赖性磷酸化。我们的研究表明,As 通过降低能量产生、改变组蛋白乙酰化和损害细胞连接而导致男性生殖毒性。我们的研究结果为进一步研究砷的生殖毒性提供了基础数据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号