Environment International ( IF 11.8 ) Pub Date : 2021-10-18 , DOI: 10.1016/j.envint.2021.106940 Mira Flasch 1 , Christoph Bueschl 2 , Giorgia Del Favero 1 , Gerhard Adam 3 , Rainer Schuhmacher 4 , Doris Marko 1 , Benedikt Warth 1
|
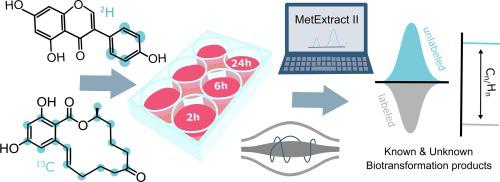
Environmental exposure to xenoestrogens, i.e., chemicals that imitate the hormone 17β-estradiol, has the potential to influence hormone homeostasis and action. Detailed knowledge of xenobiotic biotransformation processes in cell models is key when transferring knowledge learned from in vitro models to in vivo relevance. This study elucidated the metabolism of two naturally-occurring phyto- and mycoestrogens; namely genistein and zearalenone, in an estrogen receptor positive breast cancer cell line (MCF-7) with the aid of stable isotope-assisted metabolomics and the bioinformatic tool MetExtract II. Metabolism was studied in a time course experiment after 2 h, 6 h and 24 h incubation. Twelve and six biotransformation products of zearalenone and genistein were detected, respectively, clearly demonstrating the abundant xenobiotic biotransformation capability of the cells. Zearalenone underwent extensive phase-I metabolism resulting in α-zearalenol (α-ZEL), a molecule known to possess a significantly higher estrogenicity, and several phase-II metabolites (sulfo- and glycoconjugates) of the native compound and the major phase I metabolite α-ZEL. Moreover, potential adducts of zearalenone with a vitamin and several hydroxylated metabolites were annotated. Genistein metabolism resulted in sulfation, combined sulfation and hydroxylation, acetylation, glucuronidation and unexpectedly adduct formation with pentose- and hexose sugars. Kinetics of metabolite formation and subsequent excretion into the extracellular medium revealed a time-dependent increase in most biotransformation products. The untargeted elucidation of biotransformation products formed during cell culture experiments enables an improved and more meaningful interpretation of toxicological assays and has the potential to identify unexpected or unknown metabolites.
中文翻译:

通过非靶向稳定同位素辅助质谱法阐明乳腺癌细胞中的异种雌激素代谢
环境暴露于异雌激素,即模拟激素 17β-雌二醇的化学物质,具有影响激素稳态和作用的潜力。将从体外模型中学到的知识转移到体内时,细胞模型中外源生物转化过程的详细知识是关键关联。这项研究阐明了两种天然存在的植物雌激素和真菌雌激素的代谢。即染料木黄酮和玉米赤霉烯酮,在稳定同位素辅助代谢组学和生物信息学工具 MetExtract II 的帮助下,在雌激素受体阳性乳腺癌细胞系 (MCF-7) 中进行研究。在孵育 2 小时、6 小时和 24 小时后,在时间过程实验中研究代谢。分别检测到 12 种和 6 种玉米赤霉烯酮和染料木黄酮的生物转化产物,清楚地表明了细胞丰富的外源生物转化能力。玉米赤霉烯酮经历广泛的 I 期代谢,产生 α-玉米赤霉烯醇 (α-ZEL),一种已知具有显着更高雌激素性的分子,以及天然化合物和主要 I 期代谢物的几种 II 期代谢物(磺基和糖缀合物) α-ZEL。而且,注释了玉米赤霉烯酮与维生素和几种羟基化代谢物的潜在加合物。金雀异黄素代谢导致硫酸化、联合硫酸化和羟基化、乙酰化、葡糖醛酸化以及意外地与戊糖和己糖形成加合物。代谢物形成和随后排泄到细胞外介质中的动力学揭示了大多数生物转化产物的时间依赖性增加。对细胞培养实验过程中形成的生物转化产物的非靶向阐明能够对毒理学分析进行改进和更有意义的解释,并有可能识别出意外或未知的代谢物。硫酸化和羟基化、乙酰化、葡糖醛酸化以及与戊糖和己糖意外地形成加合物。代谢物形成和随后排泄到细胞外介质中的动力学揭示了大多数生物转化产物的时间依赖性增加。对细胞培养实验过程中形成的生物转化产物的非靶向阐明能够对毒理学分析进行改进和更有意义的解释,并有可能识别出意外或未知的代谢物。硫酸化和羟基化、乙酰化、葡糖醛酸化以及与戊糖和己糖意外地形成加合物。代谢物形成和随后排泄到细胞外介质中的动力学揭示了大多数生物转化产物的时间依赖性增加。对细胞培养实验过程中形成的生物转化产物的非靶向阐明能够对毒理学分析进行改进和更有意义的解释,并有可能识别出意外或未知的代谢物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号