Journal of Catalysis ( IF 7.3 ) Pub Date : 2021-10-19 , DOI: 10.1016/j.jcat.2021.10.017 Árni Björn Höskuldsson 1 , Ebrahim Tayyebi 1 , Egill Skúlason 1, 2
|
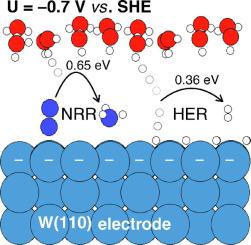
Elucidating the elementary kinetics of nitrogen reduction on transition metal surfaces can guide the search for materials capable of efficiently catalysing electrochemical ammonia synthesis at ambient conditions. Density functional theory calculations are used here to explore the elementary kinetics of nitrogen reduction and hydrogen evolution on W(110). All proton-electron transfer barriers are calculated at −0.7 V vs. SHE, where the cathodic potential is explicitly modelled using a solvation bilayer of water with hydronium ions. The protonation of adsorbed NH is determined to be rate-limiting for nitrogen reduction, with a barrier of 0.65 eV. The same reaction step is potential-limiting based on the thermochemistry of adsorbed intermediates only, showing that thermochemical calculations suffice to predict catalytic trends for nitrogen reduction. The largest barrier found for hydrogen evolution at high hydrogen coverage is 0.36 eV. Thus, hydrogen evolution is expected to dominate over nitrogen reduction on W(110) at negative potentials.
中文翻译:

钨电极电化学氮还原和析氢动力学的计算检验
阐明过渡金属表面氮还原的基本动力学可以指导寻找能够在环境条件下有效催化电化学氨合成的材料。这里使用密度泛函理论计算来探索 W(110) 上氮还原和析氢的基本动力学。所有质子-电子转移势垒均在 -0.7 V vs. SHE 下计算,其中阴极电位使用具有水合氢离子的水的溶剂化双层明确建模。吸附的 NH 的质子化被确定为氮还原的限速器,势垒为 0.65 eV。相同的反应步骤仅基于吸附中间体的热化学进行电位限制,表明热化学计算足以预测氮还原的催化趋势。在高氢覆盖率下发现的最大析氢势垒是 0.36 eV。因此,在负电位下,预计氢析出将超过 W(110) 上的氮还原。


























 京公网安备 11010802027423号
京公网安备 11010802027423号