Journal of Advanced Research ( IF 10.7 ) Pub Date : 2021-10-18 , DOI: 10.1016/j.jare.2021.10.004 Jing Guo 1 , Ying Xu 1 , Li-Jie Chen 1 , Song-Xia Zhang 1 , Yu-Ligh Liou 1 , Xiao-Ping Chen 1 , Zhi-Rong Tan 1 , Hong-Hao Zhou 1 , Wei Zhang 1 , Yao Chen 1
|
Introduction
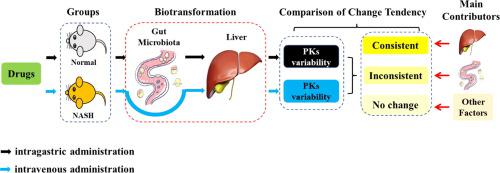
Pharmacokinetic variability in disease state is common in clinical practice, but its underlying mechanism remains unclear. Recently, gut microbiota has been considered to be pharmacokinetically equivalent to the host liver. Although some studies have explored the roles of gut microbiota and host Cyp450s in drug pharmacokinetics, few have explored their effects on pharmacokinetic variability, especially in disease states.
Objectives
In this study, we aim to investigate the effects of gut microbiota and host Cyp450s on pharmacokinetic variability in mice with non-alcoholic steatohepatitis (NASH), and to elucidate the contribution of gut microbiota and host Cyp450s to pharmacokinetic variability in this setting.
Methods
The pharmacokinetic variability of mice with NASH was explored under intragastric and intravenous administrations of a cocktail mixture of omeprazole, phenacetin, midazolam, tolbutamide, chlorzoxazone, and metoprolol, after which the results were compared with those obtained from the control group. Thereafter, the pharmacokinetic variabilities of all drugs and their relations to the changes in gut microbiota and host Cyp450s were compared and analyzed.
Results
The exposures of all drugs, except metoprolol, significantly increased in the NASH group under intragastric administration. However, no significant increase in the exposure of all drugs, except tolbutamide, was observed in the NASH group under intravenous administration. The pharmacokinetic variabilities of phenacetin, midazolam, omeprazole, and chlorzoxazone were mainly associated with decreased elimination activity in the gut microbiota. By contrast, the pharmacokinetic variability of tolbutamide was mainly related to the change in the host Cyp2c65. Notably, gut microbiota and host Cyp450s exerted minimal effects on the pharmacokinetic variability of metoprolol.
Conclusion
Gut microbiota and host Cyp450s co-contribute to the pharmacokinetic variability in mice with NASH, and the degree of contribution varies from drug to drug. The present findings provide new insights into the explanation of pharmacokinetic variability in disease states.
中文翻译:

肠道微生物群和宿主 Cyp450 共同促成了非酒精性脂肪性肝炎小鼠的药代动力学变异性:不同药物的影响各不相同
介绍
疾病状态的药代动力学变异性在临床实践中很常见,但其潜在机制仍不清楚。最近,肠道微生物群被认为在药代动力学上与宿主肝脏相当。尽管一些研究探讨了肠道微生物群和宿主 Cyp450 在药物药代动力学中的作用,但很少有人探讨它们对药代动力学变异性的影响,尤其是在疾病状态下。
目标
在这项研究中,我们旨在研究肠道微生物群和宿主 Cyp450s 对非酒精性脂肪性肝炎 (NASH) 小鼠药代动力学变异性的影响,并阐明肠道微生物群和宿主 Cyp450s 在这种情况下对药代动力学变异性的贡献。
方法
研究了在胃内和静脉内给予奥美拉唑、非那西丁、咪达唑仑、甲苯磺丁脲、氯唑沙宗和美托洛尔混合物的情况下 NASH 小鼠的药代动力学变异性,然后将结果与对照组的结果进行比较。此后,对所有药物的药代动力学变异性及其与肠道菌群和宿主 Cyp450 变化的关系进行了比较和分析。
结果
除美托洛尔外,所有药物的暴露量在 NASH 组胃内给药均显着增加。然而,在 NASH 组静脉给药下,除甲苯磺丁脲外,所有药物的暴露量均未显着增加。非那西丁、咪达唑仑、奥美拉唑和氯唑沙宗的药代动力学差异主要与肠道微生物群的消除活性降低有关。相比之下,甲苯磺丁脲的药代动力学变异性主要与宿主 Cyp2c65 的变化有关。值得注意的是,肠道微生物群和宿主 Cyp450 对美托洛尔的药代动力学变异性影响很小。
结论
肠道微生物群和宿主 Cyp450 共同促成 NASH 小鼠的药代动力学变异性,并且贡献程度因药物而异。目前的研究结果为解释疾病状态的药代动力学变异性提供了新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号