当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbohydrate Sulfation As a Mechanism for Fine-Tuning Siglec Ligands
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-10-18 , DOI: 10.1021/acschembio.1c00501 Jaesoo Jung, Jhon R. Enterina, Duong T. Bui, Fahima Mozaneh, Po-Han Lin, Nitin, Chu-Wei Kuo, Emily Rodrigues, Abhishek Bhattacherjee, Parisa Raeisimakiani, Gour C. Daskhan, Chris D. St. Laurent, Kay-Hooi Khoo, Lara K. Mahal, Wesley F. Zandberg, Xuefei Huang, John S. Klassen, Matthew S. Macauley
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-10-18 , DOI: 10.1021/acschembio.1c00501 Jaesoo Jung, Jhon R. Enterina, Duong T. Bui, Fahima Mozaneh, Po-Han Lin, Nitin, Chu-Wei Kuo, Emily Rodrigues, Abhishek Bhattacherjee, Parisa Raeisimakiani, Gour C. Daskhan, Chris D. St. Laurent, Kay-Hooi Khoo, Lara K. Mahal, Wesley F. Zandberg, Xuefei Huang, John S. Klassen, Matthew S. Macauley
|
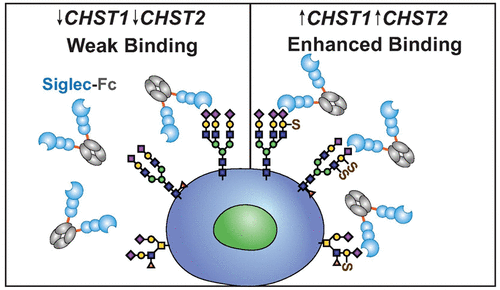
The immunomodulatory family of Siglecs recognizes sialic acid-containing glycans as “self”, which is exploited in cancer for immune evasion. The biochemical nature of Siglec ligands remains incompletely understood, with emerging evidence suggesting the importance of carbohydrate sulfation. Here, we investigate how specific sulfate modifications affect Siglec ligands by overexpressing eight carbohydrate sulfotransferases (CHSTs) in five cell lines. Overexpression of three CHSTs─CHST1, CHST2, or CHST4─significantly enhance the binding of numerous Siglecs. Unexpectedly, two other CHSTs (Gal3ST2 and Gal3ST3) diminish Siglec binding, suggesting a new mode to modulate Siglec ligands via sulfation. Results are cell type dependent, indicating that the context in which sulfated glycans are presented is important. Moreover, a pharmacological blockade of N- and O-glycan maturation reveals a cell-type-specific pattern of importance for either class of glycan. Production of a highly homogeneous Siglec-3 (CD33) fragment enabled a mass-spectrometry-based binding assay to determine ≥8-fold and ≥2-fold enhanced affinity for Neu5Acα2–3(6-O-sulfo)Galβ1–4GlcNAc and Neu5Acα2–3Galβ1–4(6-O-sulfo)GlcNAc, respectively, over Neu5Acα2–3Galβ1–4GlcNAc. CD33 shows significant additivity in affinity (≥28-fold) for the disulfated ligand, Neu5Acα2–3(6-O-sulfo)Galβ1–4(6-O-sulfo)GlcNAc. Moreover, joint overexpression of CHST1 with CHST2 in cells greatly enhanced the binding of CD33 and several other Siglecs. Finally, we reveal that CHST1 is upregulated in numerous cancers, correlating with poorer survival rates and sodium chlorate sensitivity for the binding of Siglecs to cancer cell lines. These results provide new insights into carbohydrate sulfation as a general mechanism for tuning Siglec ligands on cells, including in cancer.
中文翻译:

碳水化合物硫酸化作为微调 Siglec 配体的机制
Siglecs 的免疫调节家族将含唾液酸的聚糖识别为“自我”,它在癌症中被用于免疫逃避。Siglec 配体的生化性质仍未完全了解,新出现的证据表明碳水化合物硫酸化的重要性。在这里,我们通过在五个细胞系中过表达八种碳水化合物磺基转移酶 (CHST) 来研究特定的硫酸盐修饰如何影响 Siglec 配体。三个 CHST——CHST1、CHST2 或 CHST4——的过表达显着增强了许多 Siglecs 的结合。出乎意料的是,另外两个 CHST(Gal3ST2 和 Gal3ST3)减少了 Siglec 的结合,表明一种通过硫酸化调节 Siglec 配体的新模式。结果取决于细胞类型,表明硫酸化聚糖出现的环境很重要。此外, N - 和O的药理学阻断-聚糖成熟揭示了任一类型聚糖的细胞类型特异性模式。生产高度均一的 Siglec-3 (CD33) 片段使基于质谱的结合测定能够确定对 Neu5Acα2–3(6- O-磺基)Galβ1–4GlcNAc 和 Neu5Acα2的 ≥8 倍和 ≥2 倍增强的亲和力–3Galβ1–4(6- O -sulfo )GlcNAc,分别超过 Neu5Acα2–3Galβ1–4GlcNAc。CD33 对二硫酸化配体 Neu5Acα2–3(6- O -sulfo )Galβ1–4(6 - O ) 显示出显着的亲和力相加性(≥28 倍)-磺基)GlcNAc。此外,CHST1 与 CHST2 在细胞中的联合过表达大大增强了 CD33 和其他几个 Siglecs 的结合。最后,我们揭示了 CHST1 在许多癌症中上调,这与较差的存活率和对 Siglecs 与癌细胞系结合的氯酸钠敏感性相关。这些结果为将碳水化合物硫酸化作为调节细胞(包括癌症)上 Siglec 配体的一般机制提供了新的见解。
更新日期:2021-11-19
中文翻译:

碳水化合物硫酸化作为微调 Siglec 配体的机制
Siglecs 的免疫调节家族将含唾液酸的聚糖识别为“自我”,它在癌症中被用于免疫逃避。Siglec 配体的生化性质仍未完全了解,新出现的证据表明碳水化合物硫酸化的重要性。在这里,我们通过在五个细胞系中过表达八种碳水化合物磺基转移酶 (CHST) 来研究特定的硫酸盐修饰如何影响 Siglec 配体。三个 CHST——CHST1、CHST2 或 CHST4——的过表达显着增强了许多 Siglecs 的结合。出乎意料的是,另外两个 CHST(Gal3ST2 和 Gal3ST3)减少了 Siglec 的结合,表明一种通过硫酸化调节 Siglec 配体的新模式。结果取决于细胞类型,表明硫酸化聚糖出现的环境很重要。此外, N - 和O的药理学阻断-聚糖成熟揭示了任一类型聚糖的细胞类型特异性模式。生产高度均一的 Siglec-3 (CD33) 片段使基于质谱的结合测定能够确定对 Neu5Acα2–3(6- O-磺基)Galβ1–4GlcNAc 和 Neu5Acα2的 ≥8 倍和 ≥2 倍增强的亲和力–3Galβ1–4(6- O -sulfo )GlcNAc,分别超过 Neu5Acα2–3Galβ1–4GlcNAc。CD33 对二硫酸化配体 Neu5Acα2–3(6- O -sulfo )Galβ1–4(6 - O ) 显示出显着的亲和力相加性(≥28 倍)-磺基)GlcNAc。此外,CHST1 与 CHST2 在细胞中的联合过表达大大增强了 CD33 和其他几个 Siglecs 的结合。最后,我们揭示了 CHST1 在许多癌症中上调,这与较差的存活率和对 Siglecs 与癌细胞系结合的氯酸钠敏感性相关。这些结果为将碳水化合物硫酸化作为调节细胞(包括癌症)上 Siglec 配体的一般机制提供了新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号