当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modular Enantioselective Synthesis of cis-Cyclopropanes through Self-Sensitized Stereoselective Photodecarboxylation with Benzothiazolines
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-10-18 , DOI: 10.1021/acscatal.1c03949 Matteo Costantini 1 , Abraham Mendoza 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-10-18 , DOI: 10.1021/acscatal.1c03949 Matteo Costantini 1 , Abraham Mendoza 1
Affiliation
|
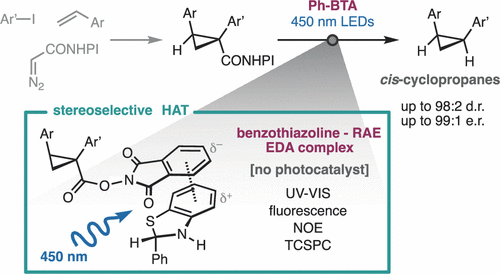
Chiral cis-cyclopropanes are strained rigid analogues of alkyl chains, whose study and application are limited by their difficult synthesis. A modular approach from olefin materials is enabled by the discovery of the electron donor–acceptor (EDA) interaction between 2-substituted benzothiazolines and N-hydroxyphthalimide esters. These complexes are activated by visible light without photocatalysts, and the benzothiazoline reagent plays a triple role as a photoreductant, a stereoselective hydrogen-atom donor, and a Brønsted acid. Beyond the enantioselective synthesis of cis-cyclopropanes, these results introduce benzothiazolines as accessible and easily tunable self-sensitized photoreductants.
中文翻译:

通过苯并噻唑啉的自敏立体选择性光脱羧反应模块化对映选择性合成顺式环丙烷
手性顺式环丙烷是烷基链的紧张刚性类似物,其研究和应用受到其合成困难的限制。通过发现 2-取代苯并噻唑啉和N-羟基邻苯二甲酰亚胺酯之间的电子供体-受体 (EDA) 相互作用,使烯烃材料的模块化方法成为可能。这些配合物在没有光催化剂的情况下被可见光活化,苯并噻唑啉试剂作为光还原剂、立体选择性氢原子供体和布朗斯台德酸发挥三重作用。除了顺式环丙烷的对映选择性合成之外,这些结果还引入了苯并噻唑啉作为可接近且易于调节的自敏光还原剂。
更新日期:2021-11-05
中文翻译:

通过苯并噻唑啉的自敏立体选择性光脱羧反应模块化对映选择性合成顺式环丙烷
手性顺式环丙烷是烷基链的紧张刚性类似物,其研究和应用受到其合成困难的限制。通过发现 2-取代苯并噻唑啉和N-羟基邻苯二甲酰亚胺酯之间的电子供体-受体 (EDA) 相互作用,使烯烃材料的模块化方法成为可能。这些配合物在没有光催化剂的情况下被可见光活化,苯并噻唑啉试剂作为光还原剂、立体选择性氢原子供体和布朗斯台德酸发挥三重作用。除了顺式环丙烷的对映选择性合成之外,这些结果还引入了苯并噻唑啉作为可接近且易于调节的自敏光还原剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号