The Journal of Supercritical Fluids ( IF 3.9 ) Pub Date : 2021-10-17 , DOI: 10.1016/j.supflu.2021.105448 Yuhang Yang 1, 2 , Liang Zhao 1, 2 , Jun Zhang 3 , Runzhou Huang 1, 2
|
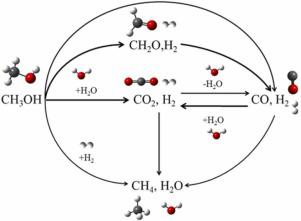
Experimental analysis and quantitative kinetic modeling were performed on methanol gasification in supercritical water using continuous reactor at different reaction temperatures (550–650 ℃), pressures (21–25 MPa), residence times (6–60 s), and initial methanol concentrations (0.05–0.2 mol L−1) to investigate the mechanism underlying methanol gasification and conversion. The kinetic model consisted of eight separate reaction pathways. Simultaneously, reaction rate and sensitivity analyses were conducted. Higher temperatures, longer residence times and lower feedstock concentrations were beneficial for the yields of H2 and CO2. Kinetic analysis showed that H2 and CO2 generation mainly occurred through the hydrolysis of methanol. CO was mainly consumed during the forward water-gas shift reaction, and the production of CH4 depended primarily on methanol methanation. Additionally, methanol hydrolysis was identified as an important elementary reaction for CH4 production by sensitivity analysis.
中文翻译:

超临界水中甲醇气化操作参数分析及动力学建模
使用连续反应器在不同反应温度(550-650 ℃)、压力(21-25 MPa)、停留时间(6-60 s)和初始甲醇浓度( 0.05–0.2 mol L -1 ) 以研究甲醇气化和转化的机理。动力学模型由八个独立的反应途径组成。同时进行反应速率和敏感性分析。较高的温度、较长的停留时间和较低的原料浓度有利于 H 2和 CO 2的产率。动力学分析表明,H 2和 CO 2主要通过甲醇水解产生。CO主要在正向水煤气变换反应过程中消耗,CH 4的生产主要依赖于甲醇甲烷化。此外,通过敏感性分析,甲醇水解被确定为 CH 4生产的重要元素反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号