当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unlocking Asymmetric Michael Additions in an Archetypical Class I Aldolase by Directed Evolution
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-10-15 , DOI: 10.1021/acscatal.1c03911 Andreas Kunzendorf 1 , Guangcai Xu 1 , Jesse J H van der Velde 1 , Henriëtte J Rozeboom 2 , Andy-Mark W H Thunnissen 2 , Gerrit J Poelarends 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-10-15 , DOI: 10.1021/acscatal.1c03911 Andreas Kunzendorf 1 , Guangcai Xu 1 , Jesse J H van der Velde 1 , Henriëtte J Rozeboom 2 , Andy-Mark W H Thunnissen 2 , Gerrit J Poelarends 1
Affiliation
|
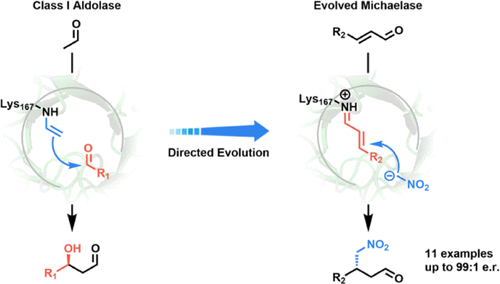
Class I aldolases catalyze asymmetric aldol addition reactions and have found extensive application in the biocatalytic synthesis of chiral β-hydroxy-carbonyl compounds. However, the usefulness of these powerful enzymes for application in other C–C bond-forming reactions remains thus far unexplored. The redesign of class I aldolases to expand their catalytic repertoire to include non-native carboligation reactions therefore continues to be a major challenge. Here, we report the successful redesign of 2-deoxy-d-ribose-5-phosphate aldolase (DERA) from Escherichia coli, an archetypical class I aldolase, to proficiently catalyze enantioselective Michael additions of nitromethane to α,β-unsaturated aldehydes to yield various pharmaceutically relevant chiral synthons. After 11 rounds of directed evolution, the redesigned DERA enzyme (DERA-MA) carried 12 amino-acid substitutions and had an impressive 190-fold enhancement in catalytic activity compared to the wildtype enzyme. The high catalytic efficiency of DERA-MA for this abiological reaction makes it a proficient “Michaelase” with potential for biocatalytic application. Crystallographic analysis provides a structural context for the evolved activity. Whereas an aldolase acts naturally by activating the enzyme-bound substrate as a nucleophile (enamine-based mechanism), DERA-MA instead acts by activating the enzyme-bound substrate as an electrophile (iminium-based mechanism). This work demonstrates the power of directed evolution to expand the reaction scope of natural aldolases to include asymmetric Michael addition reactions and presents opportunities to explore iminium catalysis with DERA-derived catalysts inspired by developments in the organocatalysis field.
中文翻译:

通过定向进化解锁原型 I 类醛缩酶中的非对称迈克尔加成
I 类醛缩酶催化不对称醛醇加成反应,并已广泛应用于手性 β-羟基-羰基化合物的生物催化合成。然而,这些强大的酶在其他 C-C 键形成反应中的应用迄今仍未得到探索。因此,重新设计 I 类醛缩酶以扩大其催化库以包括非天然碳化反应仍然是一个重大挑战。这里,我们报告的2-脱氧成功重新设计d -核糖-5-磷酸醛缩酶(DERA)从大肠杆菌,一种典型的 I 类醛缩酶,可有效催化硝基甲烷与 α,β-不饱和醛的对映选择性迈克尔加成反应,以产生各种药学相关的手性合成子。经过 11 轮定向进化后,重新设计的 DERA 酶 (DERA-MA) 进行了 12 个氨基酸置换,与野生型酶相比,催化活性提高了 190 倍。DERA-MA 对这种非生物反应的高催化效率使其成为具有生物催化应用潜力的熟练“迈克尔酶”。晶体学分析为进化活动提供了结构背景。而醛缩酶通过激活酶结合底物作为亲核试剂(基于烯胺的机制)来自然发挥作用,相反,DERA-MA 通过激活酶结合底物作为亲电试剂(亚胺基机制)起作用。这项工作证明了定向进化将天然醛缩酶的反应范围扩大到包括不对称迈克尔加成反应的能力,并提供了在有机催化领域发展的启发下探索使用 DERA 衍生催化剂进行亚胺催化的机会。
更新日期:2021-11-05
中文翻译:

通过定向进化解锁原型 I 类醛缩酶中的非对称迈克尔加成
I 类醛缩酶催化不对称醛醇加成反应,并已广泛应用于手性 β-羟基-羰基化合物的生物催化合成。然而,这些强大的酶在其他 C-C 键形成反应中的应用迄今仍未得到探索。因此,重新设计 I 类醛缩酶以扩大其催化库以包括非天然碳化反应仍然是一个重大挑战。这里,我们报告的2-脱氧成功重新设计d -核糖-5-磷酸醛缩酶(DERA)从大肠杆菌,一种典型的 I 类醛缩酶,可有效催化硝基甲烷与 α,β-不饱和醛的对映选择性迈克尔加成反应,以产生各种药学相关的手性合成子。经过 11 轮定向进化后,重新设计的 DERA 酶 (DERA-MA) 进行了 12 个氨基酸置换,与野生型酶相比,催化活性提高了 190 倍。DERA-MA 对这种非生物反应的高催化效率使其成为具有生物催化应用潜力的熟练“迈克尔酶”。晶体学分析为进化活动提供了结构背景。而醛缩酶通过激活酶结合底物作为亲核试剂(基于烯胺的机制)来自然发挥作用,相反,DERA-MA 通过激活酶结合底物作为亲电试剂(亚胺基机制)起作用。这项工作证明了定向进化将天然醛缩酶的反应范围扩大到包括不对称迈克尔加成反应的能力,并提供了在有机催化领域发展的启发下探索使用 DERA 衍生催化剂进行亚胺催化的机会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号