Science of the Total Environment ( IF 9.8 ) Pub Date : 2021-10-16 , DOI: 10.1016/j.scitotenv.2021.151005 Jiao Tang 1 , Junxiang Tang 2 , Hao Lei 3 , Yong Chen 2 , Jiang Zhao 2 , Xiaoqiang Wang 1 , Ning Pan 4
|
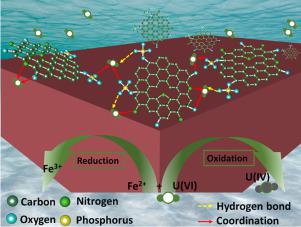
In this study, a novel, high surface area iron phosphonate (IP) for highly efficient adsorption of uranyl ion in acidic medium was described. The as-prepared IP was amorphous with its specific surface area and total pore volume as high as 268 m2/g and 1.04 cm3/g, respectively. Particularly, the as-prepared IP with ferrous ions and oxygen, nitrogen-bearing functional groups prove excellent U(VI) adsorption capacity (154.6 mg/g) as compared to that of amorphous FePO4 (67.3 mg/g) and Fe3(PO4)2(H2O)8 (33.8 mg/g). Surprising, the saturation adsorption capacity could achieve up to 353.9 mg/g. Besides, the IP also had a fast adsorption rate for attaining adsorption equilibrium within 20 min, and followed pseudo-second-order kinetic and Freundlich models. Moreover, both the Dubinin-Radushkevich isotherm adsorption model and the value of enthalpy indicated a chemisorption process. Otherwise, the Na+-independent U(VI) adsorption on IP and the adsorption-desorption isotherm studies revealed that inner-layer surface complexation is the control step for U(VI) adsorption process, and the adsorbent featured an irreversible adsorption process. The structure and functional groups of the adsorbent remained unchanged after capture of U(VI). Further, X-ray photoelectron spectra (XPS) analysis demonstrated that the capture mechanism of U(VI) on IP from acidic aqueous solution was due to not only redox reaction, but also ascribed to the coordinated chemical adsorption.
中文翻译:

用于从酸性溶液中高效捕获 U(VI) 的膦酸铁
在这项研究中,描述了一种新型的、高表面积的膦酸铁 (IP),用于在酸性介质中高效吸附铀酰离子。所制备的 IP 是无定形的,其比表面积和总孔体积分别高达 268 m 2 /g 和 1.04 cm 3 /g。特别是,与无定形 FePO 4 (67.3 mg/g) 和 Fe 3 ( PO 4 ) 2 (H 2 O) 8(33.8 毫克/克)。令人惊讶的是,饱和吸附容量可以达到 353.9 mg/g。此外,IP 还具有在 20 分钟内达到吸附平衡的快速吸附速率,并遵循伪二级动力学和 Freundlich 模型。此外,Dubinin-Radushkevich 等温吸附模型和焓值均表明化学吸附过程。否则,Na +独立的 U(VI) 吸附 IP 和吸附-解吸等温线研究表明,内层表面络合是 U(VI) 吸附过程的控制步骤,吸附剂具有不可逆的吸附过程。吸附剂的结构和官能团在捕获 U(VI) 后保持不变。此外,X射线光电子能谱(XPS)分析表明,酸性水溶液中U(VI)在IP上的捕获机制不仅是由于氧化还原反应,而且还归因于配位化学吸附。


























 京公网安备 11010802027423号
京公网安备 11010802027423号