Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Endogenous Copper for Nanocatalytic Oxidative Damage and Self-Protection Pathway Breakage of Cancer
ACS Nano ( IF 17.1 ) Pub Date : 2021-10-15 , DOI: 10.1021/acsnano.1c05451 Yuedong Guo 1, 2 , Yingying Xu 1, 2 , Qunqun Bao 3 , Chao Shen 4 , Dalong Ni 5 , Ping Hu 1, 3 , Jianlin Shi 1, 3
ACS Nano ( IF 17.1 ) Pub Date : 2021-10-15 , DOI: 10.1021/acsnano.1c05451 Yuedong Guo 1, 2 , Yingying Xu 1, 2 , Qunqun Bao 3 , Chao Shen 4 , Dalong Ni 5 , Ping Hu 1, 3 , Jianlin Shi 1, 3
Affiliation
|
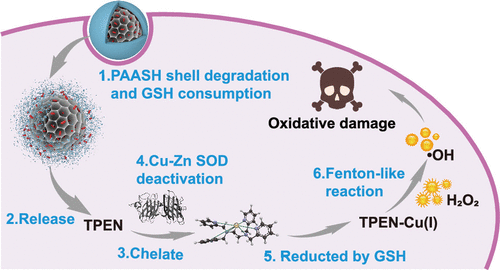
Nanocatalytic medicine is one of the most recent advances in the development of nanomedicine, which catalyzes intratumoral chemical reactions to produce toxins such as reactive oxygen species in situ for cancer specific treatment by using exogenous-delivered catalysts such as Fenton agents. However, the overexpression of reductive glutathione and Cu–Zn superoxide dismutase in cancer cells will significantly counteract the therapeutic efficacy by reactive oxygen species-mediated oxidative damages. Additionally, the direct delivery of iron-based Fenton agents may arouse undesired detrimental effects such as anaphylactic reactions. In this study, instead of exogenously delivering Fenton agents, the endogenous copper ions from intracellular Cu–Zn superoxide dismutase have been employed as the source of Fenton-like agents by chelating the Cu ions from the superoxide dismutase using a common metal ion chelator, N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine (TPEN), followed by the TPEN-Cu(II) chelate reduction to TPEN-Cu(I) by reductive glutathione. Briefly, TPEN was loaded in a disulfide bond-containing link poly(acrylic acid) shell-coated hybrid mesoporous silica/organosilicate (MSN@MON) nanocomposite as a reductive glutathione-responsive nanoplatform, which features inter-related triple functions: intratumoral reductive glutathione-responsive link poly(acrylic acid) disruption and TPEN release; the accompanying reductive glutathione consumption and Cu–Zn superoxide dismutase deactivation by TPEN chelating Cu ions from this superoxide dismutase; and the Fenton reaction catalyzed by TPEN-Cu(I) chelate as a Fenton-like agent generated from TPEN-Cu(II) reduction by the remaining reductive glutathione in cancer cells, thereby cutting off the self-protection pathway of cancer cells under severe oxidation stress and ensuring cancer cell apoptosis by reactive oxygen species produced by the catalytic Fenton-like reactions. Such a nanocatalyst demonstrates excellent biosafety and augmented therapeutic efficacy by simultaneous nanocatalytic oxidative damage and intrinsic protection pathway breakage of cancer cells.
中文翻译:

用于纳米催化氧化损伤和癌症自我保护途径断裂的内源性铜
纳米催化医学是纳米医学发展的最新进展之一,它催化肿瘤内的化学反应,原位产生活性氧等毒素通过使用外源递送的催化剂如芬顿剂进行癌症特异性治疗。然而,还原性谷胱甘肽和铜锌超氧化物歧化酶在癌细胞中的过度表达将显着抵消活性氧介导的氧化损伤的治疗效果。此外,直接递送铁基芬顿剂可能会引起不希望的有害影响,例如过敏反应。在这项研究中,通过使用普通金属离子螯合剂N螯合超氧化物歧化酶中的铜离子,细胞内铜锌超氧化物歧化酶的内源性铜离子被用作芬顿样试剂的来源,而不是外源性递送芬顿剂。, N , N ', N'-四(2-吡啶基甲基)-1,2-乙二胺 (TPEN),然后通过还原性谷胱甘肽将 TPEN-Cu(II) 螯合物还原为 TPEN-Cu(I)。简而言之,将 TPEN 负载在含二硫键的连接聚(丙烯酸)壳包覆的杂化介孔二氧化硅/有机硅酸盐(MSN@MON)纳米复合材料中作为还原性谷胱甘肽响应纳米平台,其具有相关的三重功能:肿瘤内还原性谷胱甘肽-响应性链接聚(丙烯酸)中断和 TPEN 释放;通过 TPEN 螯合来自该超氧化物歧化酶的 Cu 离子,伴随的还原性谷胱甘肽消耗和 Cu-Zn 超氧化物歧化酶失活;以及由 TPEN-Cu(I) 螯合物催化的芬顿反应,作为芬顿样试剂,由癌细胞中剩余的还原性谷胱甘肽还原 TPEN-Cu(II) 产生,从而切断癌细胞在严重氧化应激下的自我保护途径,并通过催化类芬顿反应产生的活性氧确保癌细胞凋亡。这种纳米催化剂通过同时纳米催化氧化损伤和癌细胞的内在保护途径破坏表现出优异的生物安全性和增强的治疗功效。
更新日期:2021-10-26
中文翻译:

用于纳米催化氧化损伤和癌症自我保护途径断裂的内源性铜
纳米催化医学是纳米医学发展的最新进展之一,它催化肿瘤内的化学反应,原位产生活性氧等毒素通过使用外源递送的催化剂如芬顿剂进行癌症特异性治疗。然而,还原性谷胱甘肽和铜锌超氧化物歧化酶在癌细胞中的过度表达将显着抵消活性氧介导的氧化损伤的治疗效果。此外,直接递送铁基芬顿剂可能会引起不希望的有害影响,例如过敏反应。在这项研究中,通过使用普通金属离子螯合剂N螯合超氧化物歧化酶中的铜离子,细胞内铜锌超氧化物歧化酶的内源性铜离子被用作芬顿样试剂的来源,而不是外源性递送芬顿剂。, N , N ', N'-四(2-吡啶基甲基)-1,2-乙二胺 (TPEN),然后通过还原性谷胱甘肽将 TPEN-Cu(II) 螯合物还原为 TPEN-Cu(I)。简而言之,将 TPEN 负载在含二硫键的连接聚(丙烯酸)壳包覆的杂化介孔二氧化硅/有机硅酸盐(MSN@MON)纳米复合材料中作为还原性谷胱甘肽响应纳米平台,其具有相关的三重功能:肿瘤内还原性谷胱甘肽-响应性链接聚(丙烯酸)中断和 TPEN 释放;通过 TPEN 螯合来自该超氧化物歧化酶的 Cu 离子,伴随的还原性谷胱甘肽消耗和 Cu-Zn 超氧化物歧化酶失活;以及由 TPEN-Cu(I) 螯合物催化的芬顿反应,作为芬顿样试剂,由癌细胞中剩余的还原性谷胱甘肽还原 TPEN-Cu(II) 产生,从而切断癌细胞在严重氧化应激下的自我保护途径,并通过催化类芬顿反应产生的活性氧确保癌细胞凋亡。这种纳米催化剂通过同时纳米催化氧化损伤和癌细胞的内在保护途径破坏表现出优异的生物安全性和增强的治疗功效。



























 京公网安备 11010802027423号
京公网安备 11010802027423号