当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrolysis Mechanism of the Linkers by Matrix Metalloproteinase-9 Using QM/MM Calculations
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-10-14 , DOI: 10.1021/acs.jcim.1c00825 Binbin Chen 1 , Zhengzhong Kang 1 , En Zheng 1 , Yingchun Liu 1 , James W Gauld 2 , Qi Wang 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-10-14 , DOI: 10.1021/acs.jcim.1c00825 Binbin Chen 1 , Zhengzhong Kang 1 , En Zheng 1 , Yingchun Liu 1 , James W Gauld 2 , Qi Wang 1
Affiliation
|
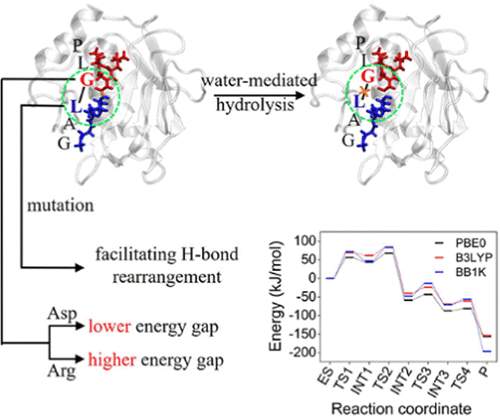
Activatable cell-penetrating peptides (ACPPs) are known to be able to decrease the cytotoxicity of cell-penetrating peptide (CPP)-based drug delivery systems. Furthermore, they can improve the targeting of CPPs when specifically recognized and hydrolyzed by characteristic proteases. A comprehensive and profound understanding of the recognition and hydrolysis process will provide a better design of the ACPP-based drug delivery system. Previous studies have clearly described how ACPPs are recognized and bound by MMPs. However, the hydrolysis mechanism of ACPPs is still unsolved. This work focuses on a proteinase-sensitive cleavable linker of ACPPs (PLGLAG), the key structure for recognition and hydrolysis, trying to determine the mechanism by which MMP-9 hydrolyzes its substrate PLGLAG. The quantum mechanics/molecular mechanics (QM/MM) calculations herein show that MMP-9 proteolysis is a water-mediated four-step reaction. More specifically, it consists of (i) nucleophilic attack, (ii) hydrogen-bond rearrangement, (iii) proton transfer, and finally (iv) amide bond rupture. Considering the reversibility of multistep reaction, the second step (i.e., hydrogen-bond rearrangement) has the highest barrier and is the rate-limiting step in the hydrolysis of PLGLAG. The possible design and improvement of the key P1 and P1′ sites are also explored through mutations. The present results indicate that, while the mutations affect the reaction energy barriers and the rate-limiting steps, all mutants considered could be hydrolyzed by MMP-9. To provide further insights, the hydrolysis mechanism of MMP-2, which has a similar hydrolysis process to that of MMP-9 but with different reaction barriers, is also studied and compared. As a result, this work provides detailed insights into the hydrolysis mechanism of ACPPs by MMP-9 and, thus, also possible insights for the development of new strategies for ACPP-based delivery systems.
中文翻译:

使用 QM/MM 计算的 Matrix Metalloproteinase-9 对接头的水解机制
已知可激活的细胞穿透肽 (ACPP) 能够降低基于细胞穿透肽 (CPP) 的药物递送系统的细胞毒性。此外,当被特征蛋白酶特异性识别和水解时,它们可以提高 CPP 的靶向性。对识别和水解过程的全面深入理解将为基于 ACPP 的药物递送系统提供更好的设计。以前的研究已经清楚地描述了 ACPPs 如何被 MMPs 识别和结合。然而,ACPPs的水解机理仍未得到解决。这项工作的重点是 ACPPs (PLGLAG) 的蛋白酶敏感可裂解接头,这是识别和水解的关键结构,试图确定 MMP-9 水解其底物 PLGLAG 的机制。本文的量子力学/分子力学 (QM/MM) 计算表明 MMP-9 蛋白水解是水介导的四步反应。更具体地说,它包括(i)亲核攻击,(ii)氢键重排,(iii)质子转移,最后(iv)酰胺键断裂。考虑到多步反应的可逆性,第二步(即氢键重排)具有最高的势垒,是PLGLAG水解的限速步骤。还通过突变探索了关键 P1 和 P1' 位点的可能设计和改进。目前的结果表明,虽然突变影响反应能垒和限速步骤,但所有考虑的突变体都可以被 MMP-9 水解。为了提供进一步的见解,MMP-2 的水解机制,MMP-9 的水解过程与 MMP-9 相似,但反应势垒不同,也进行了研究和比较。因此,这项工作提供了对 MMP-9 对 ACPP 水解机制的详细见解,因此也为基于 ACPP 的递送系统开发新策略提供了可能的见解。
更新日期:2021-10-25
中文翻译:

使用 QM/MM 计算的 Matrix Metalloproteinase-9 对接头的水解机制
已知可激活的细胞穿透肽 (ACPP) 能够降低基于细胞穿透肽 (CPP) 的药物递送系统的细胞毒性。此外,当被特征蛋白酶特异性识别和水解时,它们可以提高 CPP 的靶向性。对识别和水解过程的全面深入理解将为基于 ACPP 的药物递送系统提供更好的设计。以前的研究已经清楚地描述了 ACPPs 如何被 MMPs 识别和结合。然而,ACPPs的水解机理仍未得到解决。这项工作的重点是 ACPPs (PLGLAG) 的蛋白酶敏感可裂解接头,这是识别和水解的关键结构,试图确定 MMP-9 水解其底物 PLGLAG 的机制。本文的量子力学/分子力学 (QM/MM) 计算表明 MMP-9 蛋白水解是水介导的四步反应。更具体地说,它包括(i)亲核攻击,(ii)氢键重排,(iii)质子转移,最后(iv)酰胺键断裂。考虑到多步反应的可逆性,第二步(即氢键重排)具有最高的势垒,是PLGLAG水解的限速步骤。还通过突变探索了关键 P1 和 P1' 位点的可能设计和改进。目前的结果表明,虽然突变影响反应能垒和限速步骤,但所有考虑的突变体都可以被 MMP-9 水解。为了提供进一步的见解,MMP-2 的水解机制,MMP-9 的水解过程与 MMP-9 相似,但反应势垒不同,也进行了研究和比较。因此,这项工作提供了对 MMP-9 对 ACPP 水解机制的详细见解,因此也为基于 ACPP 的递送系统开发新策略提供了可能的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号