当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Concerted Differential Changes of Helical Dynamics and Packing upon Ligand Occupancy in a Bacterial Chemoreceptor
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-10-14 , DOI: 10.1021/acschembio.1c00576 Jesse B Gordon 1 , Mikaila C Hoffman 1 , Julianne M Troiano 1 , Mingshan Li 2 , Gerald L Hazelbauer 2 , Gabriela S Schlau-Cohen 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2021-10-14 , DOI: 10.1021/acschembio.1c00576 Jesse B Gordon 1 , Mikaila C Hoffman 1 , Julianne M Troiano 1 , Mingshan Li 2 , Gerald L Hazelbauer 2 , Gabriela S Schlau-Cohen 1
Affiliation
|
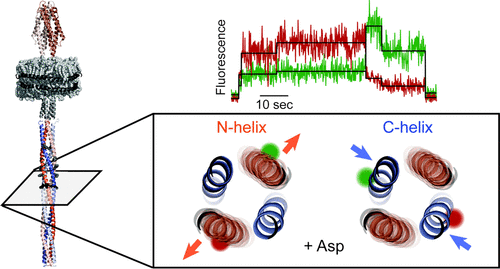
Transmembrane receptors are central components of the chemosensory systems by which motile bacteria detect and respond to chemical gradients. An attractant bound to the receptor periplasmic domain generates conformational signals that regulate a histidine kinase interacting with its cytoplasmic domain. Ligand-induced signaling through the periplasmic and transmembrane domains of the receptor involves a piston-like helical displacement, but the nature of this signaling through the >200 Å four-helix coiled coil of the cytoplasmic domain had not yet been identified. We performed single-molecule Förster resonance energy transfer measurements on Escherichia coli aspartate receptor homodimers inserted into native phospholipid bilayers enclosed in nanodiscs. The receptors were labeled with fluorophores at diagnostic positions near the middle of the cytoplasmic coiled coil. At these positions, we found that the two N-helices of the homodimer were more distant, that is, less tightly packed and more dynamic than the companion C-helix pair, consistent with previous deductions that the C-helices form a stable scaffold and the N-helices are dynamic. Upon ligand binding, the scaffold pair compacted further, while separation and dynamics of the dynamic pair increased. Thus, ligand binding had asymmetric effects on the two helical pairs, shifting mean distances in opposite directions and increasing the dynamics of one pair. We suggest that this reflects a conformational change in which differential alterations to the packing and dynamics of the two helical pairs are coupled. These coupled changes could represent a previously unappreciated mode of conformational signaling that may well occur in other coiled-coil signaling proteins.
中文翻译:

细菌化学感受器中配体占据的螺旋动力学和堆积的协同微分变化
跨膜受体是化学感应系统的核心组成部分,活动细菌通过该系统检测化学梯度并对其做出反应。与受体周质结构域结合的引诱剂产生构象信号,调节与其胞质结构域相互作用的组氨酸激酶。通过受体的周质和跨膜结构域的配体诱导信号涉及活塞样螺旋位移,但尚未确定通过细胞质结构域的 >200 Å 四螺旋螺旋的这种信号的性质。我们对大肠杆菌进行了单分子 Förster 共振能量转移测量天冬氨酸受体同型二聚体插入封闭在纳米圆盘中的天然磷脂双层中。受体在细胞质卷曲螺旋中间附近的诊断位置用荧光团标记。在这些位置,我们发现同源二聚体的两个 N-螺旋比配对的 C-螺旋对更远,即包装不那么紧密且更具活力,这与之前的推论一致,即 C-螺旋形成稳定的支架和N-螺旋是动态的。配体结合后,支架对进一步压缩,而动态对的分离和动力学增加。因此,配体结合对两个螺旋对具有不对称影响,使平均距离沿相反方向移动并增加一对的动力学。我们认为这反映了一种构象变化,其中两个螺旋对的包装和动力学的差异变化是耦合的。这些耦合变化可能代表一种以前未被重视的构象信号模式,这种模式很可能发生在其他卷曲螺旋信号蛋白中。
更新日期:2021-11-19
中文翻译:

细菌化学感受器中配体占据的螺旋动力学和堆积的协同微分变化
跨膜受体是化学感应系统的核心组成部分,活动细菌通过该系统检测化学梯度并对其做出反应。与受体周质结构域结合的引诱剂产生构象信号,调节与其胞质结构域相互作用的组氨酸激酶。通过受体的周质和跨膜结构域的配体诱导信号涉及活塞样螺旋位移,但尚未确定通过细胞质结构域的 >200 Å 四螺旋螺旋的这种信号的性质。我们对大肠杆菌进行了单分子 Förster 共振能量转移测量天冬氨酸受体同型二聚体插入封闭在纳米圆盘中的天然磷脂双层中。受体在细胞质卷曲螺旋中间附近的诊断位置用荧光团标记。在这些位置,我们发现同源二聚体的两个 N-螺旋比配对的 C-螺旋对更远,即包装不那么紧密且更具活力,这与之前的推论一致,即 C-螺旋形成稳定的支架和N-螺旋是动态的。配体结合后,支架对进一步压缩,而动态对的分离和动力学增加。因此,配体结合对两个螺旋对具有不对称影响,使平均距离沿相反方向移动并增加一对的动力学。我们认为这反映了一种构象变化,其中两个螺旋对的包装和动力学的差异变化是耦合的。这些耦合变化可能代表一种以前未被重视的构象信号模式,这种模式很可能发生在其他卷曲螺旋信号蛋白中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号