Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2021-10-14 , DOI: 10.1016/j.cej.2021.132953 Jiachen Li 1, 2 , Cong Zhang 1 , Chi Zhang 1 , Huijun Ma 3 , Yong Yang 4 , Zhaoqi Guo 1 , Yaoyu Wang 2 , Haixia Ma 1
|
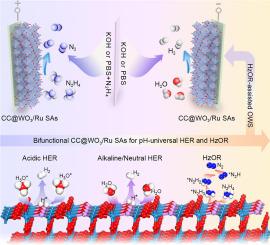
Replacing sluggish oxygen evolution reaction (OER) by hydrazine oxidation reaction (HzOR) is an energy-saving approach to assist alkaline overall water splitting (OWS). However, it is still at the infant stage as the unsatisfactory of the design strategies for bifunctional catalysts toward both HzOR and hydrogen evolution, which leads to high working potential compared to the theoretical value. Here, for the first time, we report a galvanostatic immobilization of ruthenium single atoms (Ru SAs) into the oxygen vacancy of urchin-like tungsten trioxide (Vo-WO3) and show low overpotentials of 17, 34, and 64 mV at –10 mA cm–2 under acid, alkaline, and neutral H2 evolution, respectively. As an efficient bifunctional catalyst, the Vo-WO3/Ru SAs is firstly introduced to HzOR and achieves low working potential of –58 mV at 10 mA cm–2. The HzOR-assisted OWS also delivers an ultralow cell voltage of only 25 mV at 10 mA cm–2 in alkaline medium, which is far beyond the platinum and most of the state-of-the-art catalysts. Additionally, this coupled system can also be well performed under neutral condition, realizing a cell voltage of only 137 mV at 10 mA cm–2. The excellent pH-universal HzOR-assisted OWS activity is attributed to the particular electronic configuration between Ru SAs and WO3, the immobilization of Ru SAs in WO3 could induce electron redistribution and optimize free energy of hydrogen adsorption in hydrogen evolution, while the isolation of Ru SAs also leads to the electropositivity of W sites and tends to accelerate the oxidation of hydrazine.
中文翻译:

固定在类海胆三氧化钨中的单个钌原子的电子构型在宽 pH 介质下实现肼氧化辅助析氢
用肼氧化反应(HzOR)代替缓慢的析氧反应(OER)是一种辅助碱性全水分解(OWS)的节能方法。然而,由于双功能催化剂对HzOR和析氢的设计策略并不令人满意,因此仍处于起步阶段,与理论值相比具有较高的工作潜力。在这里,我们首次报道了钌单原子(Ru SAs)在海胆状三氧化钨(Vo-WO 3)的氧空位中的恒电流固定,并显示出 17、34 和 64 mV 的低过电位 -分别在酸性、碱性和中性 H 2放出下分别为10 mA cm –2。作为一种高效的双功能催化剂,Vo-WO 3/Ru SAs 首次被引入HzOR 并在10 mA cm –2 下实现–58 mV 的低工作电位。HzOR 辅助的 OWS 还在碱性介质中在 10 mA cm –2下提供仅 25 mV 的超低电池电压,远远超过铂和大多数最先进的催化剂。此外,该耦合系统也可以在中性条件下良好运行,在 10 mA cm –2下实现仅 137 mV 的电池电压。优异的 pH 通用 HzOR 辅助 OWS 活性归因于 Ru SAs 和 WO 3之间的特殊电子构型,以及 Ru SAs 在 WO 3 中的固定 可以诱导电子重新分布并优化析氢过程中氢吸附的自由能,而 Ru SAs 的分离也导致 W 位的电正性并趋于加速肼的氧化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号