当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
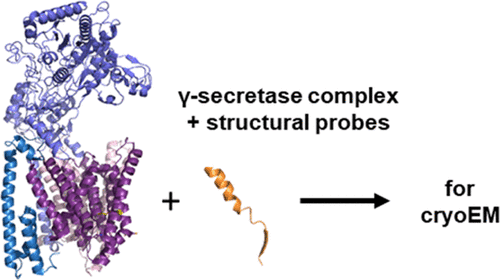
Design of Transmembrane Mimetic Structural Probes to Trap Different Stages of γ-Secretase–Substrate Interaction
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2021-10-14 , DOI: 10.1021/acs.jmedchem.1c01395 Sanjay Bhattarai 1 , Sujan Devkota 1 , Michael S Wolfe 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2021-10-14 , DOI: 10.1021/acs.jmedchem.1c01395 Sanjay Bhattarai 1 , Sujan Devkota 1 , Michael S Wolfe 1
Affiliation
|
The transmembrane domain (TMD) of the amyloid precursor protein of Alzheimer’s disease is cut processively by γ-secretase through endoproteolysis and tricarboxypeptidase “trimming”. We recently developed a prototype substrate TMD mimetic for structural analysis—composed of a helical peptide inhibitor linked to a transition-state analogue—that simultaneously engages a substrate exosite and the active site and is pre-organized to trap the carboxypeptidase transition state. Here, we developed variants of this prototype designed to allow visualization of transition states for endoproteolysis, TMD helix unwinding, and lateral gating of the substrate, identifying potent inhibitors for each class. These TMD mimetics exhibited non-competitive inhibition and occupy both the exosite and the active site, as demonstrated by inhibitor cross-competition experiments and photoaffinity probe binding assays. The new probes should be important structural tools for trapping different stages of substrate recognition and processing via ongoing cryo-electron microscopy with γ-secretase, ultimately aiding rational drug design.
中文翻译:

跨膜模拟结构探针的设计以捕获不同阶段的 γ-分泌酶-底物相互作用
阿尔茨海默病淀粉样前体蛋白的跨膜结构域 (TMD) 被 γ-分泌酶通过内切蛋白水解和三羧肽酶“修剪”逐步切割。我们最近开发了一种用于结构分析的原型底物 TMD 模拟物——由连接到过渡态类似物的螺旋肽抑制剂组成——它同时接合底物外部位点和活性位点,并预先组织以捕获羧肽酶过渡态。在这里,我们开发了这个原型的变体,旨在允许可视化内切蛋白水解、TMD 螺旋展开和底物横向门控的过渡状态,确定每个类别的有效抑制剂。这些 TMD 模拟物表现出非竞争性抑制并占据外部位点和活性位点,正如抑制剂交叉竞争实验和光亲和探针结合试验所证明的那样。新探针应该是用于捕获不同阶段的底物识别和处理的重要结构工具通过正在进行的带有γ-分泌酶的低温电子显微镜,最终有助于合理的药物设计。
更新日期:2021-10-28
中文翻译:

跨膜模拟结构探针的设计以捕获不同阶段的 γ-分泌酶-底物相互作用
阿尔茨海默病淀粉样前体蛋白的跨膜结构域 (TMD) 被 γ-分泌酶通过内切蛋白水解和三羧肽酶“修剪”逐步切割。我们最近开发了一种用于结构分析的原型底物 TMD 模拟物——由连接到过渡态类似物的螺旋肽抑制剂组成——它同时接合底物外部位点和活性位点,并预先组织以捕获羧肽酶过渡态。在这里,我们开发了这个原型的变体,旨在允许可视化内切蛋白水解、TMD 螺旋展开和底物横向门控的过渡状态,确定每个类别的有效抑制剂。这些 TMD 模拟物表现出非竞争性抑制并占据外部位点和活性位点,正如抑制剂交叉竞争实验和光亲和探针结合试验所证明的那样。新探针应该是用于捕获不同阶段的底物识别和处理的重要结构工具通过正在进行的带有γ-分泌酶的低温电子显微镜,最终有助于合理的药物设计。


























 京公网安备 11010802027423号
京公网安备 11010802027423号