当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Activation of Abl1 Kinase Explored Using Well-Tempered Metadynamics Simulations on an Essential Dynamics Sampled Path
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2021-10-14 , DOI: 10.1021/acs.jctc.1c00505 Baswanth Oruganti 1 , Ran Friedman 1
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2021-10-14 , DOI: 10.1021/acs.jctc.1c00505 Baswanth Oruganti 1 , Ran Friedman 1
Affiliation
|
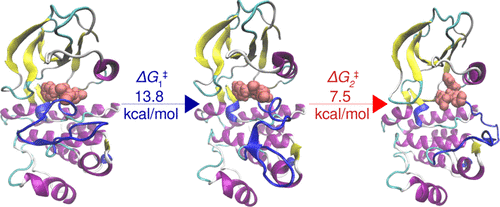
Well-tempered metadynamics (wT-metaD) simulations using path collective variables (CVs) have been successfully applied in recent years to explore conformational transitions in protein kinases and other biomolecular systems. While this methodology has the advantage of describing the transitions with a limited number of predefined path CVs, it requires as an input a reference path connecting the initial and target states of the system. It is desirable to automate the path generation using approaches that do not rely on the choice of geometric CVs to describe the transition of interest. To this end, we developed an approach that couples essential dynamics sampling with wT-metaD simulations. We used this newly developed procedure to explore the activation mechanism of Abl1 kinase and compute the associated free energy barriers. Through these simulations, we identified a three-step mechanism for the activation that involved two metastable intermediates that possessed a partially open activation loop and differed primarily in the “in” or “out” conformation of the aspartate residue of the DFG motif. One of these states is similar to a conformation that was detected in previous spectroscopic studies of Abl1 kinase, albeit its mechanistic role in the activation was hitherto not well understood. The present study establishes its intermediary role in the activation and predicts a rate-determining free energy barrier of 13.8 kcal/mol that is in good agreement with previous experimental and computational estimates. Overall, our study demonstrates the usability of essential dynamics sampling as a path CV in wT-metaD to conveniently study conformational transitions and accurately calculate the associated barriers.
中文翻译:

在基本动力学采样路径上使用温和的元动力学模拟探索 Abl1 激酶的激活
近年来,使用路径集体变量 (CV) 的温和元动力学 (wT-metaD) 模拟已成功应用于探索蛋白激酶和其他生物分子系统中的构象转变。虽然这种方法具有用有限数量的预定义路径 CV 描述转换的优点,但它需要连接系统初始状态和目标状态的参考路径作为输入。希望使用不依赖于选择几何 CV 来描述感兴趣的过渡的方法来自动生成路径。为此,我们开发了一种将基本动态采样与 wT-metaD 模拟相结合的方法。我们使用这个新开发的程序来探索 Abl1 激酶的激活机制并计算相关的自由能垒。通过这些模拟,我们确定了激活的三步机制,该机制涉及两个亚稳态中间体,它们具有部分开放的激活环,主要区别在于 DFG 基序的天冬氨酸残基的“内”或“外”构象。其中一种状态类似于先前对 Abl1 激酶的光谱研究中检测到的构象,尽管迄今为止其在激活中的机制作用尚不清楚。本研究确定了其在活化中的中介作用,并预测了 13.8 kcal/mol 的速率决定自由能垒,这与之前的实验和计算估计值非常吻合。全面的,
更新日期:2021-11-09
中文翻译:

在基本动力学采样路径上使用温和的元动力学模拟探索 Abl1 激酶的激活
近年来,使用路径集体变量 (CV) 的温和元动力学 (wT-metaD) 模拟已成功应用于探索蛋白激酶和其他生物分子系统中的构象转变。虽然这种方法具有用有限数量的预定义路径 CV 描述转换的优点,但它需要连接系统初始状态和目标状态的参考路径作为输入。希望使用不依赖于选择几何 CV 来描述感兴趣的过渡的方法来自动生成路径。为此,我们开发了一种将基本动态采样与 wT-metaD 模拟相结合的方法。我们使用这个新开发的程序来探索 Abl1 激酶的激活机制并计算相关的自由能垒。通过这些模拟,我们确定了激活的三步机制,该机制涉及两个亚稳态中间体,它们具有部分开放的激活环,主要区别在于 DFG 基序的天冬氨酸残基的“内”或“外”构象。其中一种状态类似于先前对 Abl1 激酶的光谱研究中检测到的构象,尽管迄今为止其在激活中的机制作用尚不清楚。本研究确定了其在活化中的中介作用,并预测了 13.8 kcal/mol 的速率决定自由能垒,这与之前的实验和计算估计值非常吻合。全面的,



























 京公网安备 11010802027423号
京公网安备 11010802027423号