Water Research ( IF 12.8 ) Pub Date : 2021-10-13 , DOI: 10.1016/j.watres.2021.117749 Meryem Soyluoglu 1 , Daekyun Kim 1 , Yeakub Zaker 1 , Tanju Karanfil 1
|
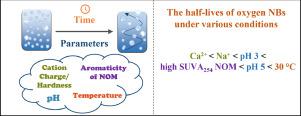
The use of nanobubbles (NBs) has gained significant attention in various applications (e.g., aeration in biological water treatment, water disinfection, membrane defouling, and ground water and sediment remediation) in recent decades because of their superior characteristics such as the improved mass transfer at the gas-liquid interfaces, their lifetime up to a couple of weeks, the formation of reactive oxygen species (ROS) with high oxidative potential. However, there is a lack of information about the effect of various factors on the stability of NBs for a long storage period under freshwater conditions. In this study, a comprehensive investigation was conducted to systematically examine the stability of oxygen NBs in water under various conditions which are closely related to a typical freshwater or the drinking water treatment. The oxygen NB stability in water was evaluated by monitoring the change in the bubble concentrations, size distribution, average diameter, and zeta potential for 60 days of storage time under different pH, hardness, ionic strength, natural organic matter (NOM), chlorine, and temperature conditions. In addition, the formation of hydroxyl radical (•OH) was investigated using disodium terephthalate which form fluorescent adducts with •OH in the presence of oxygen NBs. Among the parameters investigated, the impacts of cations, low pH, and high SUVA254 NOM on the stability of oxygen NBs were more significant than other conditions. The half-lives of oxygen NBs under various conditions follow the order Ca2+ < Na+ < pH 3 < high SUVA254 NOM < pH 5 < 30 °C. Oxygen NBs were more stable in softwater than hardwater. Oxygen NBs were relatively stable for 3 days regardless of pH. For a longer storage period, oxygen NBs disappeared faster at pH 3 than at high pH. High SUVA254 NOM destabilized NBs more than low SUVA254 NOM, indicating the impact of hydrophobicity on the NB stability. The temperature effect on the NB stability was negligible for a short storage time, while higher temperature destabilized oxygen NBs for a longer storage time. One of the main disappearance pathway of oxygen NBs in water was found to be coalescing, rising, and leaving the container, which would be promoted greatly by cations, low pH and NOM with high aromaticity. The formation of hydroxyl radical in NB solutions was detected at pH 3 by a florescent probe molecule. When oxygen NBs are released in water bodies, high calcium, high SUVA254 NOM, and low pH would significantly reduce the availability of NBs and their residence time in freshwater.
中文翻译:

淡水条件下氧纳米气泡的稳定性
近几十年来,纳米气泡 (NBs) 的使用在各种应用(例如生物水处理曝气、水消毒、膜去污、地下水和沉积物修复)中得到了广泛关注,因为它们具有改善传质等优越特性。在气液界面,它们的寿命长达几周,形成具有高氧化潜力的活性氧 (ROS)。然而,缺乏关于各种因素对 NB 在淡水条件下长期储存稳定性的影响的信息。在这项研究中,进行了一项综合调查,以系统地检查水中氧 NBs 在与典型淡水或饮用水处理密切相关的各种条件下的稳定性。通过监测不同pH、硬度、离子强度、天然有机物(NOM)、氯、和温度条件。此外,使用对苯二甲酸二钠在氧 NB 存在下与 •OH 形成荧光加合物来研究羟基自由基 (•OH) 的形成。在研究的参数中,阳离子、低 pH 值和高 SUVA 的影响 使用对苯二甲酸二钠在氧 NB 存在下与 •OH 形成荧光加合物来研究羟基自由基 (•OH) 的形成。在研究的参数中,阳离子、低 pH 值和高 SUVA 的影响 使用对苯二甲酸二钠在氧 NB 存在下与 •OH 形成荧光加合物来研究羟基自由基 (•OH) 的形成。在研究的参数中,阳离子、低 pH 值和高 SUVA 的影响254 NOM 对氧 NBs 的稳定性比其他条件更显着。不同条件下氧 NB 的半衰期遵循 Ca 2+ < Na + < pH 3 < 高 SUVA 254 NOM < pH 5 < 30 °C的顺序。氧气 NB 在软水中比硬水中更稳定。无论 pH 值如何,氧气 NBs 在 3 天内都相对稳定。对于较长的储存期,与高 pH 值相比,pH 3 下的氧 NB 消失得更快。高 SUVA 254 NOM 比低 SUVA 254更使 NB 不稳定NOM,表明疏水性对 NB 稳定性的影响。在较短的储存时间内,温度对 NB 稳定性的影响可以忽略不计,而较高的温度会使氧 NB 在较长的储存时间内不稳定。发现水中氧 NBs 的主要消失途径之一是聚结、上升和离开容器,阳离子、低 pH 值和具有高芳香性的 NOM 会大大促进这种情况。通过荧光探针分子在 pH 3 时检测到 NB 溶液中羟基自由基的形成。当氧气 NBs 在水体中释放时,高钙、高 SUVA 254 NOM 和低 pH 值会显着降低 NBs 的可用性及其在淡水中的停留时间。


























 京公网安备 11010802027423号
京公网安备 11010802027423号