European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2021-10-09 , DOI: 10.1016/j.ejmech.2021.113903 Shujing Xu 1 , Lin Sun 1 , Alexej Dick 2 , Waleed A Zalloum 3 , Tianguang Huang 1 , Megan E Meuser 2 , Xujie Zhang 1 , Yucen Tao 1 , Srinivasulu Cherukupalli 1 , Dang Ding 1 , Xiao Ding 1 , Shenghua Gao 1 , Xiangyi Jiang 1 , Dongwei Kang 1 , Erik De Clercq 4 , Christophe Pannecouque 4 , Simon Cocklin 2 , Xinyong Liu 1 , Peng Zhan 1
|
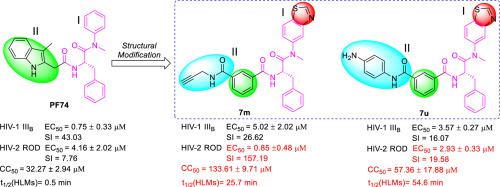
Further clinical development of PF74, a lead compound targeting HIV-1 capsid, is impeded by low antiviral activity and inferior metabolic stability. By modifying the benzene (region I) and indole of PF74, we identified two potent compounds (7m and 7u) with significantly improved metabolic stability. Compared to PF74, 7u displayed greater metabolic stability in human liver microsomes (HLMs) with half-life (t1/2) 109-fold that of PF74. Moreover, mechanism of action (MOA) studies demonstrated that 7m and 7u effectively mirrored the MOA of compounds that interact within the PF74 interprotomer pocket, showing direct and robust interactions with recombinant CA, and 7u displaying antiviral effects in both the early and late stages of HIV-1 replication. Furthermore, MD simulation corroborated that 7u was bound to the PF74 binding site, and the results of the online molinspiration software predicted that 7m and 7u had desirable physicochemical properties. Unexpectedly, this series of compounds exhibited better antiviral activity than PF74 against HIV-2, represented by compound 7m whose anti-HIV-2 activity was almost 5 times increased potency over PF74. Therefore, we have rationally redesigned the PF74 chemotype to inhibitors with novel structures and enhanced metabolic stability in this study. We hope that these new compounds can serve as a blueprint for developing a new generation of HIV treatment regimens.
中文翻译:

含有苯并噻唑部分的苯丙氨酸衍生物的设计、合成和机理研究作为 HIV-1 衣壳抑制剂,具有改善的代谢稳定性
PF74 (一种针对 HIV-1 衣壳的先导化合物)的进一步临床开发受到抗病毒活性低和代谢稳定性差的阻碍。通过修饰PF74的苯(I区)和吲哚,我们确定了两种有效的化合物(7m和7u),其代谢稳定性显着提高。与PF74相比,7u在人肝微粒体 (HLM) 中表现出更高的代谢稳定性,半衰期 (t 1/2 ) 是PF74的 109 倍。此外,作用机制 (MOA) 研究表明,7m和7u有效地反映了在PF74 原体间口袋内相互作用的化合物的 MOA ,显示出与重组 CA 的直接和稳健的相互作用,并且7u在 HIV-1 复制的早期和晚期都显示出抗病毒作用。此外,MD 模拟证实7u与PF74结合位点结合,在线 molinspiration 软件的结果预测7m和7u具有理想的理化性质。没想到这一系列化合物对HIV-2表现出比PF74更好的抗病毒活性,以化合物7m为代表其抗 HIV-2 活性几乎是PF74的 5 倍。因此,我们在本研究中合理地将PF74化学型重新设计为具有新结构和增强代谢稳定性的抑制剂。我们希望这些新化合物可以作为开发新一代 HIV 治疗方案的蓝图。


























 京公网安备 11010802027423号
京公网安备 11010802027423号