Kidney International ( IF 19.6 ) Pub Date : 2021-10-09 , DOI: 10.1016/j.kint.2021.08.031 Sian E. Piret, Ahmed A. Attallah, Xiangchen Gu, Yiqing Guo, Nehaben A. Gujarati, Justina Henein, Amy Zollman, Takashi Hato, Avi Ma’ayan, Monica P. Revelo, Kathleen G. Dickman, Chung-Hsin Chen, Chia-Tung Shun, Thomas A. Rosenquist, John C. He, Sandeep K. Mallipattu
|
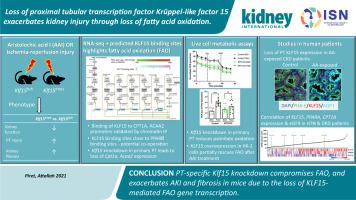
Loss of fatty acid β-oxidation (FAO) in the proximal tubule is a critical mediator of acute kidney injury and eventual fibrosis. However, transcriptional mediators of FAO in proximal tubule injury remain understudied. Krüppel-like factor 15 (KLF15), a highly enriched zinc-finger transcription factor in the proximal tubule, was significantly reduced in proximal tubule cells after aristolochic acid I (AAI) treatment, a proximal tubule-specific injury model. Proximal tubule specific knockout of Klf15 exacerbated proximal tubule injury and kidney function decline compared to control mice during the active phase of AAI treatment, and after ischemia-reperfusion injury. Furthermore, along with worsening proximal tubule injury and kidney function decline, knockout mice exhibited increased kidney fibrosis as compared to control mice during the remodeling phase after AAI treatment. RNA-sequencing of kidney cortex demonstrated increased transcripts involved in immune system and integrin signaling pathways and decreased transcripts encompassing metabolic pathways, specifically FAO, and PPARα signaling, in knockout versus control mice after AAI treatment. In silico and experimental chromatin immunoprecipitation studies collectively demonstrated that KLF15 occupied the promoter region of key FAO genes, CPT1A and ACAA2, in close proximity to transcription factor PPARα binding sites. While the loss of Klf15 reduced the expression of Cpt1a and Acaa2 and led to compromised FAO, induction of KLF15 partially rescued loss of FAO in AAI-treated cells. Klf15, Ppara, Cpt1a, and Acaa2 expression was also decreased in other mouse kidney injury models. Tubulointerstitial KLF15 independently correlated with eGFR, PPARA and CPT1A appearance in expression arrays from human kidney biopsies. Thus, proximal tubule-specific loss of Klf15 exacerbates acute kidney injury and fibrosis, likely due to loss of interaction with PPARα leading to loss of FAO gene transcription.
中文翻译:

近端肾小管转录因子 Krüppel 样因子 15 的缺失通过脂肪酸氧化的缺失加剧肾损伤
近端小管中脂肪酸 β-氧化 (FAO) 的缺失是急性肾损伤和最终纤维化的关键介质。然而,FAO 在近端小管损伤中的转录介质仍未得到充分研究。Krüppel 样因子 15 (KLF15) 是近端小管中高度富集的锌指转录因子,在马兜铃酸 I (AAI) 治疗后近端小管细胞中显着减少,这是一种近端小管特异性损伤模型。Klf15的近端小管特异性敲除在 AAI 治疗的活跃期和缺血再灌注损伤后,与对照小鼠相比,近端小管损伤和肾功能下降加剧。此外,随着近端小管损伤和肾功能下降的恶化,在 AAI 治疗后的重塑阶段,基因敲除小鼠与对照小鼠相比表现出肾纤维化增加。肾皮质的 RNA 测序表明,在 AAI 处理后的基因敲除小鼠和对照小鼠中,参与免疫系统和整合素信号通路的转录本增加,而包含代谢通路(特别是 FAO 和 PPARα 信号通路)的转录本减少。计算机和实验染色质免疫沉淀研究共同表明,KLF15 占据了关键 FAO 基因的启动子区域,CPT1A和ACAA2,靠近转录因子 PPARα 结合位点。虽然Klf15的缺失降低了Cpt1a和Acaa2的表达并导致 FAO 受损,但KLF15的诱导部分挽救了 AAI 处理细胞中 FAO 的缺失。Klf15、Ppara、Cpt1a和Acaa2表达在其他小鼠肾损伤模型中也有所降低。肾小管间质KLF15与人肾活检表达阵列中的eGFR、 PPARA和CPT1A外观独立相关。因此,近端小管特异性丢失Klf15加剧了急性肾损伤和纤维化,这可能是由于与 PPARα 相互作用的丧失导致 FAO 基因转录的丧失。


























 京公网安备 11010802027423号
京公网安备 11010802027423号