当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Isobaric Vapor–Liquid Equilibria and Extractive Distillation Process Design for Separating Ethanol and Diethoxymethane
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-10-08 , DOI: 10.1021/acs.jced.1c00443 Xiaoyan Yi 1 , Jie Kong 1 , Yunfei Song 1 , Jie Wang 1 , Lanyi Sun 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-10-08 , DOI: 10.1021/acs.jced.1c00443 Xiaoyan Yi 1 , Jie Kong 1 , Yunfei Song 1 , Jie Wang 1 , Lanyi Sun 1
Affiliation
|
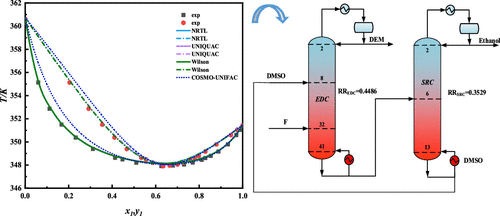
The purification of diethoxymethane (DEM) is a challenge because unreacted ethanol forms an azeotrope with DEM in the acetalization reaction of formaldehyde with ethanol to produce DEM. In this work, extractive distillation with dimethyl sulfoxide (DMSO) as an entrainer was adopted to separate the mixture of ethanol and DEM. Isobaric vapor–liquid equilibrium data of ethanol + DEM and DEM + DMSO were measured at 101.3 kPa, and the thermodynamic consistency of the experimental data was tested by the Fredenslund and van Ness test methods. The activity coefficient models, which are nonrandom two-liquid (NRTL), universal quasichemical (UNIQUAC), and Wilson models, were successfully applied to correlate the experimental data. Then, the feasibility of separating the azeotrope with DMSO as the entrainer, the operating region and the separation sequence were studied by thermodynamic topological analysis. The extractive distillation process was designed and optimized by the sequential iterative procedure based on the total annual cost (TAC). The results show that DMSO can be used to separate ethanol and DEM by extractive distillation.
中文翻译:

分离乙醇和二乙氧基甲烷的等压汽液平衡和萃取精馏工艺设计
二乙氧基甲烷 (DEM) 的纯化是一个挑战,因为在甲醛与乙醇的缩醛化反应中,未反应的乙醇会与 DEM 形成共沸物,以生产 DEM。在这项工作中,采用以二甲基亚砜 (DMSO) 为夹带剂的萃取精馏分离乙醇和 DEM 的混合物。乙醇+DEM和DEM+DMSO的等压汽液平衡数据在101.3kPa下测得,实验数据的热力学一致性通过Fredenslund和van Ness测试方法进行测试。活动系数模型,即非随机二液 (NRTL)、通用准化学 (UNIQUAC) 和威尔逊模型,已成功应用于关联实验数据。然后,以DMSO为夹带剂分离共沸物的可行性,通过热力学拓扑分析研究了工作区域和分离顺序。萃取精馏过程是根据年总成本 (TAC) 通过顺序迭代程序设计和优化的。结果表明DMSO可用于萃取精馏分离乙醇和DEM。
更新日期:2021-12-09
中文翻译:

分离乙醇和二乙氧基甲烷的等压汽液平衡和萃取精馏工艺设计
二乙氧基甲烷 (DEM) 的纯化是一个挑战,因为在甲醛与乙醇的缩醛化反应中,未反应的乙醇会与 DEM 形成共沸物,以生产 DEM。在这项工作中,采用以二甲基亚砜 (DMSO) 为夹带剂的萃取精馏分离乙醇和 DEM 的混合物。乙醇+DEM和DEM+DMSO的等压汽液平衡数据在101.3kPa下测得,实验数据的热力学一致性通过Fredenslund和van Ness测试方法进行测试。活动系数模型,即非随机二液 (NRTL)、通用准化学 (UNIQUAC) 和威尔逊模型,已成功应用于关联实验数据。然后,以DMSO为夹带剂分离共沸物的可行性,通过热力学拓扑分析研究了工作区域和分离顺序。萃取精馏过程是根据年总成本 (TAC) 通过顺序迭代程序设计和优化的。结果表明DMSO可用于萃取精馏分离乙醇和DEM。



























 京公网安备 11010802027423号
京公网安备 11010802027423号