当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Local Chemical Environment Governs Anode Processes in CO2 Electrolyzers
ACS Energy Letters ( IF 22.0 ) Pub Date : 2021-10-07 , DOI: 10.1021/acsenergylett.1c01937 Ádám Vass 1 , Balázs Endrődi 1 , Gergely Ferenc Samu 1 , Ádám Balog 1 , Attila Kormányos 1, 2 , Serhiy Cherevko 2 , Csaba Janáky 1
ACS Energy Letters ( IF 22.0 ) Pub Date : 2021-10-07 , DOI: 10.1021/acsenergylett.1c01937 Ádám Vass 1 , Balázs Endrődi 1 , Gergely Ferenc Samu 1 , Ádám Balog 1 , Attila Kormányos 1, 2 , Serhiy Cherevko 2 , Csaba Janáky 1
Affiliation
|
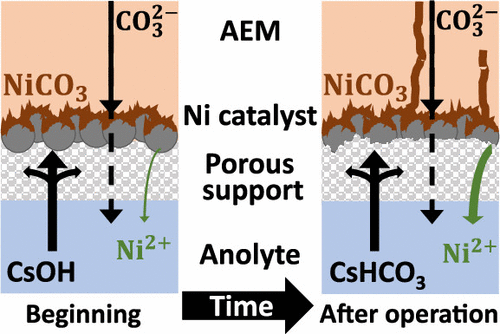
A major goal within the CO2 electrolysis community is to replace the generally used Ir anode catalyst with a more abundant material, which is stable and active for water oxidation under process conditions. Ni is widely applied in alkaline water electrolysis, and it has been considered as a potential anode catalyst in CO2 electrolysis. Here we compare the operation of electrolyzer cells with Ir and Ni anodes and demonstrate that, while Ir is stable under process conditions, the degradation of Ni leads to a rapid cell failure. This is caused by two parallel mechanisms: (i) a pH decrease of the anolyte to a near neutral value and (ii) the local chemical environment developing at the anode (i.e., high carbonate concentration). The latter is detrimental for zero-gap electrolyzer cells only, but the first mechanism is universal, occurring in any kind of CO2 electrolyzer after prolonged operation with recirculated anolyte.
中文翻译:

当地化学环境控制 CO2 电解槽中的阳极过程
CO 2电解界的一个主要目标是用更丰富的材料代替常用的 Ir 阳极催化剂,该材料在工艺条件下对水氧化具有稳定和活性。Ni广泛应用于碱性水电解,被认为是CO 2 中潜在的阳极催化剂电解。在这里,我们比较了带有 Ir 和 Ni 阳极的电解槽的运行情况,并证明虽然 Ir 在工艺条件下是稳定的,但 Ni 的降解会导致电池快速失效。这是由两种平行机制引起的:(i) 阳极电解液的 pH 值降低到接近中性值和 (ii) 在阳极处形成的局部化学环境(即高碳酸盐浓度)。后者仅对零间隙电解槽有害,但第一种机制是普遍的,在使用再循环阳极电解液长时间操作后发生在任何类型的 CO 2电解槽中。
更新日期:2021-11-12
中文翻译:

当地化学环境控制 CO2 电解槽中的阳极过程
CO 2电解界的一个主要目标是用更丰富的材料代替常用的 Ir 阳极催化剂,该材料在工艺条件下对水氧化具有稳定和活性。Ni广泛应用于碱性水电解,被认为是CO 2 中潜在的阳极催化剂电解。在这里,我们比较了带有 Ir 和 Ni 阳极的电解槽的运行情况,并证明虽然 Ir 在工艺条件下是稳定的,但 Ni 的降解会导致电池快速失效。这是由两种平行机制引起的:(i) 阳极电解液的 pH 值降低到接近中性值和 (ii) 在阳极处形成的局部化学环境(即高碳酸盐浓度)。后者仅对零间隙电解槽有害,但第一种机制是普遍的,在使用再循环阳极电解液长时间操作后发生在任何类型的 CO 2电解槽中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号