当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of Electrolyte Oxidation and Difluorophosphoric Acid Generation in Crossover and Capacity Fade in Lithium Ion Batteries
ACS Energy Letters ( IF 22.0 ) Pub Date : 2021-10-06 , DOI: 10.1021/acsenergylett.1c01657 Chamithri Jayawardana 1 , Nuwanthi Rodrigo 1 , Bharathy Parimalam 1 , Brett L Lucht 1
ACS Energy Letters ( IF 22.0 ) Pub Date : 2021-10-06 , DOI: 10.1021/acsenergylett.1c01657 Chamithri Jayawardana 1 , Nuwanthi Rodrigo 1 , Bharathy Parimalam 1 , Brett L Lucht 1
Affiliation
|
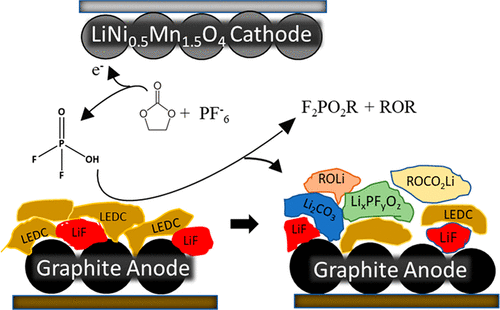
Poor cycling performance for many high voltage lithium ion batteries (LIB) has been attributed to damage of the anode solid electrolyte interphase (SEI) resulting from crossover reactions. Transition-metal ion crossover has been proposed as a primary source of SEI damage and capacity loss, especially for high-voltage spinel cathodes. However, deposition of transition metals on the anode SEI may not be the primary source of SEI degradation. This investigation focuses on the oxidative decomposition of LiPF6 in ethylene carbonate (EC)-based carbonate electrolytes to generate acidic species which subsequently cross over to the anode and degrade the anode SEI components. The generation of the strong acid, difluorophosphoric acid (F2PO2H), has been quantified for both graphite || LiNi0.5Mn1.5O4 and graphite || LiMn2O4 cells. There is a correlation between the concentration F2PO2H, SEI degradation, and the capacity loss of the cells.
中文翻译:

电解液氧化和二氟磷酸生成在锂离子电池交叉和容量衰减中的作用
许多高压锂离子电池 (LIB) 的循环性能不佳归因于交叉反应导致阳极固体电解质中间相 (SEI) 的损坏。过渡金属离子交叉被认为是 SEI 损坏和容量损失的主要来源,尤其是对于高压尖晶石阴极。然而,过渡金属在阳极 SEI 上的沉积可能不是 SEI 降解的主要来源。该研究的重点是 LiPF 6在基于碳酸亚乙酯 (EC) 的碳酸盐电解质中的氧化分解,以生成酸性物质,随后这些物质会转移到阳极并降解阳极 SEI 组分。生成强酸二氟磷酸(F 2 PO 2H), 已被量化为石墨 || LiNi 0.5 Mn 1.5 O 4和石墨 || LiMn 2 O 4电池。F 2 PO 2 H浓度、SEI 降解和电池容量损失之间存在相关性。
更新日期:2021-11-12
中文翻译:

电解液氧化和二氟磷酸生成在锂离子电池交叉和容量衰减中的作用
许多高压锂离子电池 (LIB) 的循环性能不佳归因于交叉反应导致阳极固体电解质中间相 (SEI) 的损坏。过渡金属离子交叉被认为是 SEI 损坏和容量损失的主要来源,尤其是对于高压尖晶石阴极。然而,过渡金属在阳极 SEI 上的沉积可能不是 SEI 降解的主要来源。该研究的重点是 LiPF 6在基于碳酸亚乙酯 (EC) 的碳酸盐电解质中的氧化分解,以生成酸性物质,随后这些物质会转移到阳极并降解阳极 SEI 组分。生成强酸二氟磷酸(F 2 PO 2H), 已被量化为石墨 || LiNi 0.5 Mn 1.5 O 4和石墨 || LiMn 2 O 4电池。F 2 PO 2 H浓度、SEI 降解和电池容量损失之间存在相关性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号