当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Reductive Cross-Coupling of Heteroaryl Chlorides and Aryl Chlorides
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-10-06 , DOI: 10.1021/acscatal.1c02307 Bijan Mirabi 1 , Austin D. Marchese 1 , Mark Lautens 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-10-06 , DOI: 10.1021/acscatal.1c02307 Bijan Mirabi 1 , Austin D. Marchese 1 , Mark Lautens 1
Affiliation
|
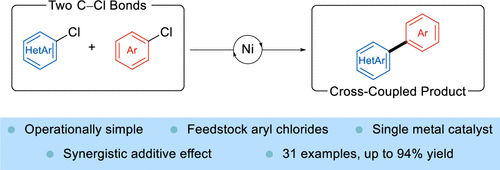
We report a nickel-catalyzed cross-electrophile coupling reaction of aryl chlorides and heteroaryl chlorides enabled by a synergistic combination consisting of halide effects and the addition of a magnesium salt. The reaction relies on the electronic difference between the aromatic and heteroaromatic coupling partners to afford the cross-coupled biaryl products using a single catalyst. A variety of heterocycles were amenable to the reaction, as well as a wide range of aryl chlorides, with electron-deficient aryl chlorides performing the best in the reaction. Preliminary mechanistic evidence demonstrates the MgCl2 is essential to the reaction by accelerating the reduction of Ni(II), and that small quantities of iodide lead to improved yields.
中文翻译:

镍催化的杂芳基氯化物和芳基氯化物的还原交叉偶联
我们报告了镍催化的芳基氯化物和杂芳基氯化物的交叉亲电偶联反应,该反应通过由卤化物效应和添加镁盐组成的协同组合实现。该反应依赖于芳香族和杂芳香族偶联伙伴之间的电子差异,以使用单一催化剂提供交叉偶联的联芳基产物。各种杂环以及各种芳基氯化物都适用于该反应,其中缺电子的芳基氯化物在反应中表现最好。初步机理证据表明,MgCl 2通过加速 Ni(II) 的还原对反应至关重要,并且少量碘化物可提高产率。
更新日期:2021-10-15
中文翻译:

镍催化的杂芳基氯化物和芳基氯化物的还原交叉偶联
我们报告了镍催化的芳基氯化物和杂芳基氯化物的交叉亲电偶联反应,该反应通过由卤化物效应和添加镁盐组成的协同组合实现。该反应依赖于芳香族和杂芳香族偶联伙伴之间的电子差异,以使用单一催化剂提供交叉偶联的联芳基产物。各种杂环以及各种芳基氯化物都适用于该反应,其中缺电子的芳基氯化物在反应中表现最好。初步机理证据表明,MgCl 2通过加速 Ni(II) 的还原对反应至关重要,并且少量碘化物可提高产率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号