当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phosphorus-Driven Electron Delocalization on Edge-Type FeN4 Active Sites for Oxygen Reduction in Acid Medium
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-10-05 , DOI: 10.1021/acscatal.1c02259 Hengbo Yin 1 , Pengfei Yuan 2 , Bang-An Lu 1 , Huicong Xia 1 , Kai Guo 1 , Gege Yang 1 , Gan Qu 1 , Dongping Xue 1 , Yongfeng Hu 3 , Junqi Cheng 1 , Shichun Mu 4, 5 , Jia-Nan Zhang 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-10-05 , DOI: 10.1021/acscatal.1c02259 Hengbo Yin 1 , Pengfei Yuan 2 , Bang-An Lu 1 , Huicong Xia 1 , Kai Guo 1 , Gege Yang 1 , Gan Qu 1 , Dongping Xue 1 , Yongfeng Hu 3 , Junqi Cheng 1 , Shichun Mu 4, 5 , Jia-Nan Zhang 1
Affiliation
|
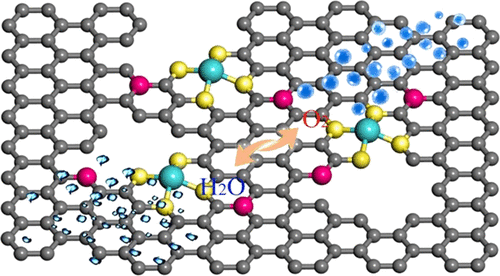
Precise tuning of the chemical environment of neighboring atomic FeN4 sites is extremely important for optimizing Fe–N–C catalysts to produce the fast oxygen reduction reaction (ORR) kinetics both in acidic and alkaline media, but it is actually very challenging. Heteroatoms could affect the metal charge of the active center through long-range electron delocalization; however, there are a few studies on it. Herein, density functional theory (DFT) calculations demonstrate that the addition of long-range P into edge-type FeN4 can drive the electron delocalization and decrease the band gap of the FeN4 center, leading to a substantial decrease in the free energy barrier to direct four-electron ORR kinetics compared to P-free edge-type FeN4, indicating superior intrinsic ORR activity. Experimentally, by incorporating P in edge-rich FeN4 supported on N,P-doped carbon (Fe–N–C–P/N,P–C), the created Fe–N–C catalyst presents the greatly increased acidic ORR activity, with a half-wave potential (E1/2) of 0.80 V (vs a reversible hydrogen electrode), which approaches that of commercial Pt/C and also has a high half-wave potential of 0.87 V, beyond Pt/C for alkaline ORR. In addition, it shows higher proton exchange membrane fuel cell and Zn-air battery performances than the pristine Fe–C–N catalyst (Fe–N–C/N–C). This work will guide the rational design of highly active metal atomic scale catalysts with optimized chemical surroundings in terms of P incorporation as a chemically tunable method.
中文翻译:

在酸性介质中用于还原氧的边缘型 FeN4 活性位点上的磷驱动电子离域化
精确调整相邻原子 FeN 4位点的化学环境对于优化 Fe-N-C 催化剂以在酸性和碱性介质中产生快速氧还原反应 (ORR) 动力学极其重要,但实际上非常具有挑战性。杂原子可以通过长程电子离域影响活性中心的金属电荷;然而,有一些关于它的研究。在此,密度泛函理论 (DFT) 计算表明,在边缘型 FeN 4 中加入长程 P可以驱动电子离域并降低 FeN 4中心的带隙,从而导致自由能垒的显着降低与无 P 边缘型 FeN 4相比,指导四电子 ORR 动力学,表明优越的内在 ORR 活性。实验上,通过将 P 掺入负载在 N,P 掺杂碳 (Fe–N–C–P/N,P–C) 上的富边缘 FeN 4 中,所产生的 Fe–N–C 催化剂表现出大大提高的酸性 ORR 活性,半波电位 ( E 1/2 ) 为 0.80 V(相对于可逆氢电极),接近商用 Pt/C 的半波电位,并且还具有 0.87 V 的高半波电位,超过 Pt/C碱性 ORR。此外,它显示出比原始 Fe-C-N 催化剂(Fe-N-C/N-C)更高的质子交换膜燃料电池和锌空气电池性能。这项工作将指导合理设计具有优化化学环境的高活性金属原子级催化剂,将 P 掺入作为一种化学可调方法。
更新日期:2021-10-15
中文翻译:

在酸性介质中用于还原氧的边缘型 FeN4 活性位点上的磷驱动电子离域化
精确调整相邻原子 FeN 4位点的化学环境对于优化 Fe-N-C 催化剂以在酸性和碱性介质中产生快速氧还原反应 (ORR) 动力学极其重要,但实际上非常具有挑战性。杂原子可以通过长程电子离域影响活性中心的金属电荷;然而,有一些关于它的研究。在此,密度泛函理论 (DFT) 计算表明,在边缘型 FeN 4 中加入长程 P可以驱动电子离域并降低 FeN 4中心的带隙,从而导致自由能垒的显着降低与无 P 边缘型 FeN 4相比,指导四电子 ORR 动力学,表明优越的内在 ORR 活性。实验上,通过将 P 掺入负载在 N,P 掺杂碳 (Fe–N–C–P/N,P–C) 上的富边缘 FeN 4 中,所产生的 Fe–N–C 催化剂表现出大大提高的酸性 ORR 活性,半波电位 ( E 1/2 ) 为 0.80 V(相对于可逆氢电极),接近商用 Pt/C 的半波电位,并且还具有 0.87 V 的高半波电位,超过 Pt/C碱性 ORR。此外,它显示出比原始 Fe-C-N 催化剂(Fe-N-C/N-C)更高的质子交换膜燃料电池和锌空气电池性能。这项工作将指导合理设计具有优化化学环境的高活性金属原子级催化剂,将 P 掺入作为一种化学可调方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号