Gastrointestinal Endoscopy ( IF 7.7 ) Pub Date : 2021-10-05 , DOI: 10.1016/j.gie.2021.09.035 Sung Hyun Cho 1 , Tae Jun Song 1 , Dong-Wan Seo 1 , Dongwook Oh 1 , Do Hyun Park 1 , Sang Soo Lee 1 , Sung Koo Lee 1 , Myung-Hwan Kim 1
|
Background and Aims
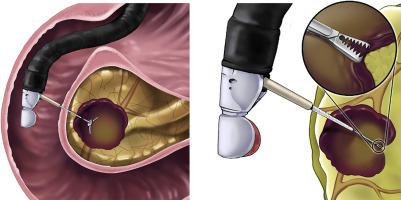
EUS-guided through-the-needle biopsy sampling (EUS-TTNB) using microbiopsy forceps is performed for the accurate diagnosis of pancreatic cystic lesions (PCLs). However, there are no standardized protocols for this procedure, and the amount of data on its efficacy is limited. Here, we evaluated the feasibility, efficacy, and safety of EUS-TTNB in categorizing the types of PCLs and identified the factors associated with diagnostic failure.
Methods
The prospectively collected and maintained EUS-TTNB database at Asan Medical Center was reviewed to identify patients with PCLs who underwent EUS-TTNB between January 2019 and January 2021. The primary outcomes were technical success, diagnostic yield, and adverse events. Factors contributing to diagnostic failure and the discrepancies in the diagnosis made by conventional modalities (ie, EUS morphology, cross-sectional imaging, and cystic fluid analysis) were also evaluated.
Results
Forty-five patients were analyzed. EUS-TTNB was successfully performed in all patients (technical success, 100%). Histologic diagnosis of PCLs was made in 37 patients (diagnostic yield, 82%). When comparing EUS-TTNB with a presumptive diagnosis, EUS-TTNB changed the diagnosis in 10 patients in terms of the categorization of the types of PCLs. The diagnostic yield was significantly higher in those who had 4 or more visible biopsy specimens per session (93%) than in those with fewer than 4 visible biopsy specimens per session (67%; P = .045). During follow-up, 3 patients (7%) experienced adverse events (2 acute pancreatitis, 1 intracystic bleeding), and no life-threatening adverse event occurred.
Conclusions
EUS-TTNB showed high technical feasibility, diagnostic yield, and good safety profile. EUS-TTNB may improve the categorization of the types of PCLs. Studies with standardized procedure protocols are needed to reduce the diagnostic failure for the types of PCLs.
中文翻译:

EUS引导下经针穿刺活检取样在胰腺囊性病变类型分类中的有效性和安全性
背景和目标
使用微活检钳进行 EUS 引导的穿刺活检取样 (EUS-TTNB) 以准确诊断胰腺囊性病变 (PCL)。然而,这个过程没有标准化的协议,关于其功效的数据量是有限的。在这里,我们评估了 EUS-TTNB 对 PCL 类型进行分类的可行性、有效性和安全性,并确定了与诊断失败相关的因素。
方法
回顾了在牙山医疗中心前瞻性收集和维护的 EUS-TTNB 数据库,以确定在 2019 年 1 月至 2021 年 1 月期间接受 EUS-TTNB 的 PCL 患者。主要结果是技术成功、诊断率和不良事件。还评估了导致诊断失败的因素和传统方式(即 EUS 形态学、横截面成像和囊液分析)诊断中的差异。
结果
分析了 45 名患者。EUS-TTNB 在所有患者中均成功进行(技术成功率为 100%)。37 名患者进行了 PCL 的组织学诊断(诊断率为 82%)。在将 EUS-TTNB 与推定诊断进行比较时,EUS-TTNB 根据 PCL 类型的分类改变了 10 名患者的诊断。每次有 4 个或更多可见活检标本的患者 (93%) 的诊断率显着高于每次可见活检标本少于 4 个的患者 (67%; P = .045)。随访期间,3例(7%)患者出现不良事件(2例急性胰腺炎,1例囊内出血),未发生危及生命的不良事件。
结论
EUS-TTNB 显示出很高的技术可行性、诊断率和良好的安全性。EUS-TTNB 可以改进 PCL 类型的分类。需要使用标准化程序协议进行研究,以减少 PCL 类型的诊断失败。


























 京公网安备 11010802027423号
京公网安备 11010802027423号