European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2021-10-06 , DOI: 10.1016/j.ejmech.2021.113888 Yiyue Feng 1 , Yingmei Lu 1 , Junfang Li 1 , Honghua Zhang 1 , Zhao Li 1 , Hanzhong Feng 2 , Xuemei Deng 1 , Dan Liu 1 , Tao Shi 1 , Weifan Jiang 3 , Yongxing He 2 , Jian Zhang 4 , Zhen Wang 5
|
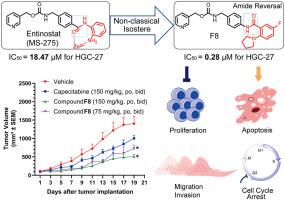
Although gastric cancer has become a major public health problem, oral agents applied in clinics for gastric cancer therapy are scarce. Therefore, to explore new oral chemical entities with high efficiency and low toxicity, 41 o-aminobenzamide derivatives based on the scaffolds of MS-275 and SAHA were designed, synthesized, and evaluated for their anti-gastric cancer abilities in vitro and in vivo. Structure-activity relationships were discussed, leading to the identification of compounds F8 (IC50 = 0.28 μM against HGC-27 cell) and T9 (IC50 = 1.84 μM against HGC-27 cell) with improved cytotoxicity, anti-gastric cancer proliferation potency, induction of cell apoptosis and cell cycle arrest ability, inhibition of cell migration and invasion. What is worth mentioning is that compound F8 was more efficient and less toxic than the positive drug capecitabine in vivo on the HGC-27-xenograft model. Meanwhile, compound F8 exhibited suitable pharmacokinetic properties and less acute toxicity (LD50 > 1000 mg/kg). Besides, western blotting analysis, IHC analysis, differentially expressed proteins analysis and ABPP experiment indicated that compound F8 could modulate molecular pathways involved in apoptosis and cell cycle progression. Consequently, compound F8 is a strong candidate for the development of human gastric cancer therapy.
中文翻译:

新型邻氨基苯甲酰胺衍生物作为体外和体内潜在抗胃癌药物的设计、合成和生物学评价
尽管胃癌已成为主要的公共卫生问题,但临床上用于胃癌治疗的口服药物却很少。因此,为探索高效、低毒的新型口服化学实体,设计、合成了基于MS-275和SAHA支架的41个邻氨基苯甲酰胺衍生物,并在体外和体内评估了它们的抗胃癌能力。讨论了构效关系,从而鉴定出化合物 F8(IC 50 = 0.28 μM 针对 HGC-27 细胞)和 T9(IC 50 = 1.84 μM 针对 HGC-27 细胞)具有改善的细胞毒性、抗胃癌增殖能力、诱导细胞凋亡和细胞周期停滞能力、抑制细胞迁移和侵袭。值得一提的是,在HGC-27-异种移植模型中,化合物F8在体内比阳性药物卡培他滨更有效、毒性更小。同时,化合物 F8 表现出合适的药代动力学特性和较低的急性毒性(LD 50 > 1000 毫克/公斤)。此外,蛋白质印迹分析、IHC分析、差异表达蛋白分析和ABPP实验表明,化合物F8可以调节参与细胞凋亡和细胞周期进程的分子通路。因此,化合物 F8 是开发人类胃癌治疗的有力候选者。


























 京公网安备 11010802027423号
京公网安备 11010802027423号