European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2021-10-05 , DOI: 10.1016/j.ejmech.2021.113897 Jun Yan 1 , Yuzhu Xu 2 , Xing Jin 2 , Qiaoxuan Zhang 1 , Feng Ouyang 3 , Liqiao Han 3 , Min Zhan 3 , Xingshu Li 4 , Baoxia Liang 5 , Xianzhang Huang 1
|
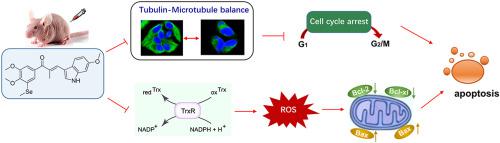
Microtubule target agents (MTAs) are widely-used clinical anti-cancer drugs for decades, but the acquired drug resistance severely restricted their application. Thioredoxin reductases (TrxR) was reported to be overexpressed in most tumors and closely related to high risk of cancer recurrence and drug resistance, making it a potential target for anticancer drug discovery. Multi-target-directed ligands (MTDLs) by a single molecule provide a logical and alternative approach to drug combinations. In this work, based on the structure-activity relationships obtained in our previous study, some structure modifications were performed. On one hand, the retained skeleton structure of MTAs endowed its tubulin polymerization inhibition activity, on the other hand, the selenium-containing structure and α,β-unsaturated ketone moiety endowed the TrxR inhibition activity. As results, the newly obtained compounds exhibited superior anti-proliferative activities towards various human cancer cells and drug-resistance cells, and displayed high selectivity towards various human normal cells. The mechanism study revealed that the dual effect of cell cycle arrest triggered by targeting tubulin and the abnormal accumulation of ROS caused by TrxR inhibition eventually lead to cell apoptosis. Notably, compared with the MTA agents CA-4P, and the TrxR inhibitor Ethaselen, the optimized compound 14c, which served as dual-targeting inhibitor of tubulin and TrxR, exerted greatly improved in vivo anti-tumor activity. In summary, 14c deserved further consideration for cancer therapy.
中文翻译:

通过双靶向微管蛋白和TrxR对吲哚查尔酮衍生物作为抗肿瘤剂的结构修饰和生物学评价
微管靶向剂(MTA)是几十年来广泛使用的临床抗癌药物,但获得性耐药严重限制了其应用。据报道,硫氧还蛋白还原酶(TrxR)在大多数肿瘤中过度表达,与癌症复发的高风险和耐药性密切相关,使其成为抗癌药物发现的潜在靶点。单个分子的多靶点定向配体 (MTDL) 为药物组合提供了一种合乎逻辑的替代方法。在这项工作中,基于我们之前研究中获得的构效关系,进行了一些结构修改。一方面,MTAs保留的骨架结构赋予其微管蛋白聚合抑制活性,另一方面,含硒结构和α,β-不饱和酮部分赋予了 TrxR 抑制活性。结果,新获得的化合物对各种人类癌细胞和耐药细胞表现出优异的抗增殖活性,并对各种人类正常细胞表现出高选择性。机制研究表明,靶向微管蛋白触发的细胞周期停滞和TrxR抑制引起的ROS异常积累的双重作用最终导致细胞凋亡。值得注意的是,与 MTA 药物 CA-4P 和 TrxR 抑制剂 Ethaselen 相比,优化的化合物 机制研究表明,靶向微管蛋白触发的细胞周期停滞和TrxR抑制引起的ROS异常积累的双重作用最终导致细胞凋亡。值得注意的是,与 MTA 药物 CA-4P 和 TrxR 抑制剂 Ethaselen 相比,优化的化合物 机制研究表明,靶向微管蛋白引发的细胞周期停滞和TrxR抑制引起的ROS异常积累的双重作用最终导致细胞凋亡。值得注意的是,与 MTA 药物 CA-4P 和 TrxR 抑制剂 Ethaselen 相比,优化的化合物14c作为微管蛋白和 TrxR 的双重靶向抑制剂,具有显着提高的体内抗肿瘤活性。总之,14c值得进一步考虑用于癌症治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号