当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structures of a dodecameric multicopper oxidase from Marinithermus hydrothermalis
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-10-04 , DOI: 10.1107/s205979832100944x Joseph L Paavola 1 , Umberto Battistin 1 , Craig M Ogata 2 , Millie M Georgiadis 1
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-10-04 , DOI: 10.1107/s205979832100944x Joseph L Paavola 1 , Umberto Battistin 1 , Craig M Ogata 2 , Millie M Georgiadis 1
Affiliation
|
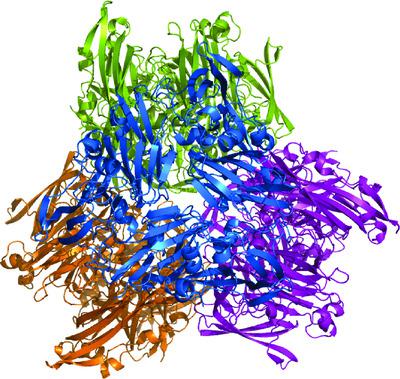
Multicopper oxidases (MCOs) represent a diverse family of enzymes that catalyze the oxidation of either an organic or a metal substrate with concomitant reduction of dioxygen to water. These enzymes contain variable numbers of cupredoxin domains, two, three or six per subunit, and rely on four copper ions, a single type I copper and three additional copper ions organized in a trinuclear cluster (TNC), with one type II and two type III copper ions, to catalyze the reaction. Here, two crystal structures and the enzymatic characterization of Marinithermus hydrothermalis MCO, a two-domain enzyme, are reported. This enzyme decolorizes Congo Red dye at 70°C in the presence of high halide concentrations and may therefore be useful in the detoxification of industrial waste that contains dyes. In two distinct crystal structures, MhMCO forms the trimers seen in other two-domain MCOs, but differs from these enzymes in that four trimers interact to create a dodecamer. This dodecamer of MhMCO forms a closed ball-like structure and has implications for the sequestration of bound divalent metal ions as well as substrate accessibility. In each subunit of the dodecameric structures, a Trp residue, Trp351, located between the type I and TNC sites exists in two distinct conformations, consistent with a potential role in facilitating electron transfer in the enzyme.
中文翻译:

一种来自 Marinithermus hydrothermalis 的十二聚体多铜氧化酶的晶体结构
多铜氧化酶 (MCO) 代表了一个多样化的酶家族,它们催化有机或金属底物的氧化,同时将分子氧还原为水。这些酶包含可变数量的铜氧还蛋白结构域,每个亚基有两个、三个或六个,并且依赖于四个铜离子、一个 I 型铜和三个额外的铜离子组成一个三核簇 (TNC),一个 II 型和两个III 铜离子,催化反应。在这里,Marinithermus hydrothermalis的两种晶体结构和酶学表征报道了MCO,一种双域酶。这种酶在 70°C 下在高卤化物浓度的情况下使刚果红染料脱色,因此可用于含有染料的工业废物的解毒。在两种不同的晶体结构中,MhMCO 形成了在其他双域 MCO 中看到的三聚体,但与这些酶的不同之处在于四个三聚体相互作用以产生十二聚体。这种 MhMCO 的十二聚体形成一个封闭的球状结构,对结合的二价金属离子的螯合以及底物的可及性具有重要意义。在十二聚体结构的每个亚基中,位于 I 型和 TNC 位点之间的 Trp 残基 Trp351 以两种不同的构象存在,这与促进酶中电子转移的潜在作用一致。
更新日期:2021-10-04
中文翻译:

一种来自 Marinithermus hydrothermalis 的十二聚体多铜氧化酶的晶体结构
多铜氧化酶 (MCO) 代表了一个多样化的酶家族,它们催化有机或金属底物的氧化,同时将分子氧还原为水。这些酶包含可变数量的铜氧还蛋白结构域,每个亚基有两个、三个或六个,并且依赖于四个铜离子、一个 I 型铜和三个额外的铜离子组成一个三核簇 (TNC),一个 II 型和两个III 铜离子,催化反应。在这里,Marinithermus hydrothermalis的两种晶体结构和酶学表征报道了MCO,一种双域酶。这种酶在 70°C 下在高卤化物浓度的情况下使刚果红染料脱色,因此可用于含有染料的工业废物的解毒。在两种不同的晶体结构中,MhMCO 形成了在其他双域 MCO 中看到的三聚体,但与这些酶的不同之处在于四个三聚体相互作用以产生十二聚体。这种 MhMCO 的十二聚体形成一个封闭的球状结构,对结合的二价金属离子的螯合以及底物的可及性具有重要意义。在十二聚体结构的每个亚基中,位于 I 型和 TNC 位点之间的 Trp 残基 Trp351 以两种不同的构象存在,这与促进酶中电子转移的潜在作用一致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号