Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A three-stage design for allergen immunotherapy trials
Allergy ( IF 12.4 ) Pub Date : 2021-10-02 , DOI: 10.1111/all.15117 Xinyu Tang 1 , Ronald L Rabin 2 , Lihan K Yan 1
Allergy ( IF 12.4 ) Pub Date : 2021-10-02 , DOI: 10.1111/all.15117 Xinyu Tang 1 , Ronald L Rabin 2 , Lihan K Yan 1
Affiliation
|
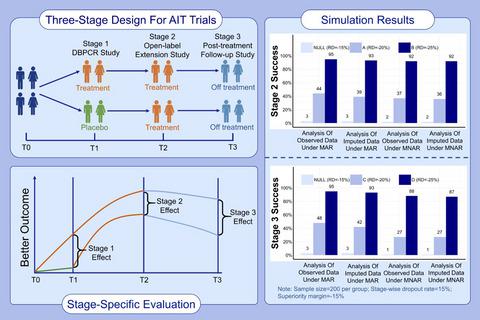
Clinical trials of allergen immunotherapy (AIT) may require up to 5 years to complete. These lengthy trials may be complicated by high and potentially differential dropouts, especially among participants who perceive that they are receiving placebo. We propose a three-stage design in which the placebo group in Stage 1 crosses over to receive active treatment in Stage 2. In Stage 3, AIT is discontinued to determine whether benefit is maintained post-treatment. We apply inferential statistics to support the three-stage design for clinical trials to determine clinical efficacy, treatment response over time, and sustained response to AIT.
中文翻译:

过敏原免疫治疗试验的三阶段设计
过敏原免疫疗法 (AIT) 的临床试验可能需要长达 5 年才能完成。这些冗长的试验可能会因高辍学率和潜在差异性辍学而变得复杂,尤其是在那些认为自己正在接受安慰剂的参与者中。我们提出了一个三阶段设计,其中第 1 阶段的安慰剂组在第 2 阶段交叉接受积极治疗。在第 3 阶段,停止 AIT 以确定治疗后是否保持益处。我们应用推论统计来支持临床试验的三阶段设计,以确定临床疗效、随时间的治疗反应和对 AIT 的持续反应。
更新日期:2021-10-02
中文翻译:

过敏原免疫治疗试验的三阶段设计
过敏原免疫疗法 (AIT) 的临床试验可能需要长达 5 年才能完成。这些冗长的试验可能会因高辍学率和潜在差异性辍学而变得复杂,尤其是在那些认为自己正在接受安慰剂的参与者中。我们提出了一个三阶段设计,其中第 1 阶段的安慰剂组在第 2 阶段交叉接受积极治疗。在第 3 阶段,停止 AIT 以确定治疗后是否保持益处。我们应用推论统计来支持临床试验的三阶段设计,以确定临床疗效、随时间的治疗反应和对 AIT 的持续反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号