European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2021-09-30 , DOI: 10.1016/j.ejmech.2021.113875 Andrea Petreni 1 , Sameh M Osman 2 , Fatmah A Alasmary 2 , Tahani M Almutairi 2 , Alessio Nocentini 1 , Claudiu T Supuran 1
|
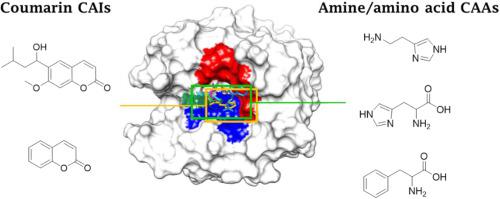
The first structural analysis comparing the binding mode to the target carbonic anhydrases (CAs, EC 4.2.1.1) of two opposite classes of modulators is presented here: coumarin derivatives act as prodrug CA inhibitors (CAIs), being hydrolyzed by the enzyme esterase activity to 2-hydroxycinnamic acids that occlude the active site entrance; CA activators (CAAs) belonging of the amine and amino acid types, enhance the CA activity by increasing the efficiency of the rate-determining proton shuttling step in the CA catalytic cycle. Analysis of the crystallographic data available for the human CA isoform II in adduct with two coumarin CAIs and some CAAs showed that both types of CA modulators bind in the same region of the enzyme active site, basically interacting with superimposable amino acid residues, that are Trp5, Asn62, His64, Asn67, Gln92, Thr200. A plethora of water molecules also participate in the adducts formation. This structural analysis showed that presence of certain chemical groups in the compound structure is mandatory to produce an activating rather than inhibitory action, such as multiple nitrogen- and oxygen-based moieties capable of shuttling protons or forming extended H-bond networks nearby the proton shuttle residue. This constitutes the only known example among all enzymes of an identical binding site for inhibitors and activators, which, in addition, possess significant pharmacological applications.
中文翻译:

人碳酸酐酶的香豆素抑制剂和胺/氨基酸激活剂的结合位点比较
第一个结构分析比较了两种相反类别的调节剂与目标碳酸酐酶 (CAs, EC 4.2.1.1) 的结合模式:香豆素衍生物充当前药 CA 抑制剂 (CAIs),被酶酯酶活性水解以封闭活性位点入口的 2-羟基肉桂酸;属于胺和氨基酸类型的 CA 活化剂 (CAA) 通过提高 CA 催化循环中决定速率的质子穿梭步骤的效率来增强 CA 活性。对人类 CA 异构体 II 与两种香豆素 CAIs 和一些 CAA 的加合物的可用晶体学数据分析表明,两种类型的 CA 调节剂结合在酶活性位点的同一区域,基本上与可叠加的氨基酸残基相互作用,即 Trp5 、Asn62、His64、Asn67、Gln92、Thr200。过多的水分子也参与了加合物的形成。该结构分析表明,化合物结构中某些化学基团的存在对于产生活化而不是抑制作用是必不可少的,例如能够穿梭质子或在质子穿梭附近形成延伸的氢键网络的多个氮和氧基部分残留物。这构成了抑制剂和活化剂具有相同结合位点的所有酶中唯一已知的例子,此外,它们还具有重要的药理学应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号