当前位置:
X-MOL 学术
›
Am. J. Transplant.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A randomized controlled trial of liposomal cyclosporine A for inhalation in the prevention of bronchiolitis obliterans syndrome following lung transplantation
American Journal of Transplantation ( IF 8.8 ) Pub Date : 2021-09-29 , DOI: 10.1111/ajt.16858 Claus Neurohr 1, 2 , Nikolaus Kneidinger 1 , Alessandro Ghiani 2 , Víctor Monforte 3 , Christiane Knoop 4 , Peter Jaksch 5 , Jasvir Parmar 6 , Piedad Ussetti 7 , Amparo Sole 8 , Joachim Müller-Quernheim 9 , Romain Kessler 10 , Hubert Wirtz 11 , Gerhard Boerner 12 , Oliver Denk 12 , Stefanie Prante Fernandes 12 , Juergen Behr 1
American Journal of Transplantation ( IF 8.8 ) Pub Date : 2021-09-29 , DOI: 10.1111/ajt.16858 Claus Neurohr 1, 2 , Nikolaus Kneidinger 1 , Alessandro Ghiani 2 , Víctor Monforte 3 , Christiane Knoop 4 , Peter Jaksch 5 , Jasvir Parmar 6 , Piedad Ussetti 7 , Amparo Sole 8 , Joachim Müller-Quernheim 9 , Romain Kessler 10 , Hubert Wirtz 11 , Gerhard Boerner 12 , Oliver Denk 12 , Stefanie Prante Fernandes 12 , Juergen Behr 1
Affiliation
|
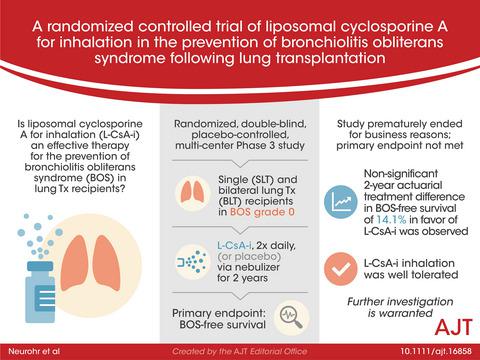
Long-term survival after lung transplantation is limited by chronic allograft dysfunction. The aim of this study was to investigate the effect of locally augmented immunosuppression with liposomal cyclosporine A for inhalation (L-CsA-i) for the prevention of bronchiolitis obliterans syndrome (BOS). In a randomized, double-blind, placebo-controlled, multi-center Phase 3 study, 180 LT recipients in BOS grade 0 were planned to receive L-CsA-i or placebo in addition to triple-drug immunosuppression. L-CsA-i was administered twice daily via an Investigational eFlow nebulizer to recipients of single (SLT) and bilateral lung transplants (BLT) within 6–32 weeks posttransplant, and continued for 2 years. The primary endpoint was BOS-free survival. 130 patients were enrolled before the study was prematurely terminated for business reasons. Despite a 2-year actuarial difference in BOS-free survival of 14.1% in favor of L-CsA-i in the overall study population, the primary endpoint was not met (p = .243). The pre-defined per protocol analysis of SLT recipients (n = 24) resulted in a treatment difference of 58.2% (p = .053). No difference was observed in the BLT (n = 48) subpopulation (p = .973). L-CsA-i inhalation was well tolerated. Although this study failed to meet its primary endpoint, the results warrant additional investigation of L-CsA-i in lung transplant recipients.
中文翻译:

吸入脂质体环孢菌素 A 预防肺移植后闭塞性细支气管炎综合征的随机对照试验
肺移植后的长期生存受到慢性同种异体移植物功能障碍的限制。本研究的目的是研究局部增强免疫抑制与脂质体环孢菌素 A 吸入 (L-CsA-i) 预防闭塞性细支气管炎综合征 (BOS) 的效果。在一项随机、双盲、安慰剂对照、多中心的 3 期研究中,180 名 BOS 0 级 LT 接受者计划在三联药物免疫抑制之外接受 L-CsA-i 或安慰剂。L-CsA-i 在移植后 6-32 周内通过研究性 eFlow 雾化器每天两次对单肺移植 (SLT) 和双侧肺移植 (BLT) 的接受者给药,并持续 2 年。主要终点是无 BOS 生存期。在研究因商业原因提前终止之前,共有 130 名患者入组。p = .243)。对 SLT 接受者 (n = 24) 的预定义方案分析导致治疗差异为 58.2% ( p = .053)。在 BLT ( n = 48) 亚群 ( p = .973) 中未观察到差异。L-CsA-i 吸入耐受性良好。尽管这项研究未能达到其主要终点,但结果值得对肺移植受者中的 L-CsA-i 进行额外调查。
更新日期:2021-09-29
中文翻译:

吸入脂质体环孢菌素 A 预防肺移植后闭塞性细支气管炎综合征的随机对照试验
肺移植后的长期生存受到慢性同种异体移植物功能障碍的限制。本研究的目的是研究局部增强免疫抑制与脂质体环孢菌素 A 吸入 (L-CsA-i) 预防闭塞性细支气管炎综合征 (BOS) 的效果。在一项随机、双盲、安慰剂对照、多中心的 3 期研究中,180 名 BOS 0 级 LT 接受者计划在三联药物免疫抑制之外接受 L-CsA-i 或安慰剂。L-CsA-i 在移植后 6-32 周内通过研究性 eFlow 雾化器每天两次对单肺移植 (SLT) 和双侧肺移植 (BLT) 的接受者给药,并持续 2 年。主要终点是无 BOS 生存期。在研究因商业原因提前终止之前,共有 130 名患者入组。p = .243)。对 SLT 接受者 (n = 24) 的预定义方案分析导致治疗差异为 58.2% ( p = .053)。在 BLT ( n = 48) 亚群 ( p = .973) 中未观察到差异。L-CsA-i 吸入耐受性良好。尽管这项研究未能达到其主要终点,但结果值得对肺移植受者中的 L-CsA-i 进行额外调查。



























 京公网安备 11010802027423号
京公网安备 11010802027423号