当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigating Tetrel-Based Neutral Frustrated Lewis Pairs for Hydrogen Activation
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2021-09-30 , DOI: 10.1021/acs.inorgchem.1c01543 Pallavi Sarkar 1 , Shubhajit Das 1 , Swapan K Pati 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2021-09-30 , DOI: 10.1021/acs.inorgchem.1c01543 Pallavi Sarkar 1 , Shubhajit Das 1 , Swapan K Pati 1
Affiliation
|
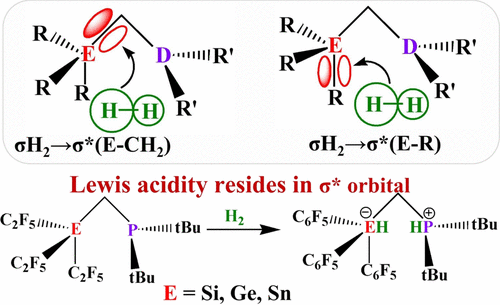
Tetrel Lewis acids are a prospective alternative to commonly employed neutral boranes in frustrated Lewis pair (FLP) chemistry. While cationic tetrylium Lewis acids, being isolobal and iso(valence)electronic, are a natural replacement to boranes, neutral tetrel Lewis acids allude as less trivial options due to the absence of a formally empty p orbital on the acceptor atom. Recently, a series of intramolecular geminal FLPs (C2F5)3E-CH2-P(tBu)2 (E = Si, Ge, Sn) featuring neutral tetrel atoms as acceptor sites has been reported for activation of small molecules including H2. In this work, through density functional theory computations, we elucidate the general mechanistic picture of H2 activation by this family of FLPs. Our findings reveal that the acceptor atom derives the required Lewis acidity utilizing the antibonding orbitals of its adjacent bonds with the individual contributions depending on the identity of the acceptor and the donor atoms. By varying the identity of the Lewis acid and Lewis base sites and attached substituents, we unravel their interplay on the energetics of the H2 activation. We find that switching the donor site from P to N significantly affects the synchronous nature of the bond breaking/formations along the reaction pathway, and as a result, N-bearing FLPs have a more favorable H2 activation profile than those with P. Our results are quantitatively discussed in detail within the framework of the activation–strain model of reactivity along with the energy-decomposition analysis method. Finally, the reductive elimination decomposition route pertinent to the plausible extension of the H2 activation to catalytic hydrogenation by these FLPs is also examined.
中文翻译:

研究基于 Tetrel 的中性受阻路易斯对用于氢活化
Tetrel Lewis 酸是受挫路易斯对 (FLP) 化学中常用中性硼烷的潜在替代品。虽然阳离子四合路易斯酸是等叶和异(价)电子的,是硼烷的天然替代品,但中性四合路易斯酸由于受体原子上不存在形式上空的 p 轨道而暗示为不太重要的选择。最近,已经报道了一系列分子内孪晶 FLPs (C 2 F 5 ) 3 E-CH 2 -P( t Bu) 2 (E = Si, Ge, Sn) 以中性四氢原子作为受体位点用于激活小分子包括H 2. 在这项工作中,通过密度泛函理论计算,我们阐明了该 FLP 家族的H 2激活的一般机制图。我们的研究结果表明,受体原子利用其相邻键的反键轨道获得所需的路易斯酸度,而个体贡献取决于受体和供体原子的身份。通过改变路易斯酸和路易斯碱位点以及连接的取代基的身份,我们解开了它们对 H 2活化能量学的相互作用。我们发现将供体位点从 P 切换到 N 会显着影响沿反应途径的键断裂/形成的同步性质,因此,含 N 的 FLP 具有更有利的 H 2激活曲线比那些与 P. 我们的结果在反应性的激活 - 应变模型以及能量分解分析方法的框架内进行了定量讨论。最后,还检查了与这些 FLP的 H 2活化到催化氢化的合理扩展相关的还原消除分解途径。
更新日期:2021-10-18
中文翻译:

研究基于 Tetrel 的中性受阻路易斯对用于氢活化
Tetrel Lewis 酸是受挫路易斯对 (FLP) 化学中常用中性硼烷的潜在替代品。虽然阳离子四合路易斯酸是等叶和异(价)电子的,是硼烷的天然替代品,但中性四合路易斯酸由于受体原子上不存在形式上空的 p 轨道而暗示为不太重要的选择。最近,已经报道了一系列分子内孪晶 FLPs (C 2 F 5 ) 3 E-CH 2 -P( t Bu) 2 (E = Si, Ge, Sn) 以中性四氢原子作为受体位点用于激活小分子包括H 2. 在这项工作中,通过密度泛函理论计算,我们阐明了该 FLP 家族的H 2激活的一般机制图。我们的研究结果表明,受体原子利用其相邻键的反键轨道获得所需的路易斯酸度,而个体贡献取决于受体和供体原子的身份。通过改变路易斯酸和路易斯碱位点以及连接的取代基的身份,我们解开了它们对 H 2活化能量学的相互作用。我们发现将供体位点从 P 切换到 N 会显着影响沿反应途径的键断裂/形成的同步性质,因此,含 N 的 FLP 具有更有利的 H 2激活曲线比那些与 P. 我们的结果在反应性的激活 - 应变模型以及能量分解分析方法的框架内进行了定量讨论。最后,还检查了与这些 FLP的 H 2活化到催化氢化的合理扩展相关的还原消除分解途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号