当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diastereo- and Enantioselective Synthesis of Eight-Membered Heterocycles via an Allylation/Ring Expansion Sequence Enabled by Multiple Catalysis
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-09-29 , DOI: 10.1021/acscatal.1c03711 Wu-Lin Yang 1 , Yuan-Lin Wang 1 , Wen Li 1 , Bu-Ming Gu 1 , Si-Wen Wang 1 , Xiaoyan Luo 1 , Bo-Xue Tian 2 , Wei-Ping Deng 1, 3
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-09-29 , DOI: 10.1021/acscatal.1c03711 Wu-Lin Yang 1 , Yuan-Lin Wang 1 , Wen Li 1 , Bu-Ming Gu 1 , Si-Wen Wang 1 , Xiaoyan Luo 1 , Bo-Xue Tian 2 , Wei-Ping Deng 1, 3
Affiliation
|
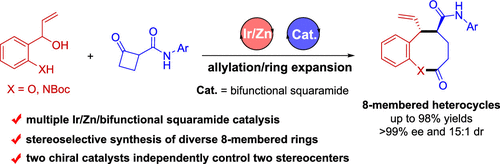
The development of protocols for constructing chiral medium-sized heterocycles with high efficiency and excellent stereocontrol is of great interest owing to their ubiquitous occurrence in natural products and biologically active pharmaceuticals. Nonetheless, current synthetic approaches are limited due to unfavorable enthalpy and entropy factors, as well as transannular interactions. The present work addresses this issue by designing an asymmetric allylation/ring expansion reaction of 2-(1-hydroxyallyl)phenols and cyclobutanone carboxamides enabled by sequential iridium/zinc/bifunctional squaramide catalysis, affording a series of 8-membered benzo[b]oxocines in high yields with high diastereo- and enantioselectivities. Mechanistic investigation reveals that the enantioselectivity is controlled by the chiral iridium catalyst, while density functional theory calculations demonstrate that the diastereoselectivity is controlled by the chiral bifunctional squaramide catalyst. Moreover, the sequential allylation reaction strategy is demonstrated to be also applicable to the synthesis of two types of enantiomerically enriched nitrogen heterocycles, 8-membered benzo[b]azocines and polycyclic cyclobuta[b]quinolines.
中文翻译:

通过多重催化的烯丙基化/环扩展序列非对映选择性和对映选择性合成八元杂环
由于它们普遍存在于天然产物和生物活性药物中,因此开发具有高效和出色立体控制的手性中等大小杂环的构建方案引起了人们的极大兴趣。尽管如此,由于不利的焓和熵因子以及跨环相互作用,当前的合成方法受到限制。目前的工作通过设计 2-(1-羟基烯丙基) 苯酚和环丁酮甲酰胺的不对称烯丙基化/扩环反应来解决这个问题,该反应由顺序铱/锌/双功能方酸酰胺催化实现,提供一系列 8 元苯并 [ b]] oxocines 高产,高非对映选择性和对映选择性。机理研究表明,对映选择性受手性铱催化剂控制,而密度泛函理论计算表明,非对映选择性受手性双功能方酸酰胺催化剂控制。此外,顺序烯丙基化反应策略也被证明适用于两种对映体富集的氮杂环,8 元苯并 [ b ] azocine 和多环环丁 [ b ] 喹啉的合成。
更新日期:2021-10-15
中文翻译:

通过多重催化的烯丙基化/环扩展序列非对映选择性和对映选择性合成八元杂环
由于它们普遍存在于天然产物和生物活性药物中,因此开发具有高效和出色立体控制的手性中等大小杂环的构建方案引起了人们的极大兴趣。尽管如此,由于不利的焓和熵因子以及跨环相互作用,当前的合成方法受到限制。目前的工作通过设计 2-(1-羟基烯丙基) 苯酚和环丁酮甲酰胺的不对称烯丙基化/扩环反应来解决这个问题,该反应由顺序铱/锌/双功能方酸酰胺催化实现,提供一系列 8 元苯并 [ b]] oxocines 高产,高非对映选择性和对映选择性。机理研究表明,对映选择性受手性铱催化剂控制,而密度泛函理论计算表明,非对映选择性受手性双功能方酸酰胺催化剂控制。此外,顺序烯丙基化反应策略也被证明适用于两种对映体富集的氮杂环,8 元苯并 [ b ] azocine 和多环环丁 [ b ] 喹啉的合成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号