Chest ( IF 9.6 ) Pub Date : 2021-09-27 , DOI: 10.1016/j.chest.2021.09.024 Christopher D Barrett 1 , Hunter B Moore 2 , Ernest E Moore 3 , Janice Wang 4 , Negin Hajizadeh 4 , Walter L Biffl 5 , Lawrence Lottenberg 6 , Purvesh R Patel 7 , Michael S Truitt 8 , Robert C McIntyre 2 , Todd M Bull 9 , Lee Anne Ammons 10 , Arsen Ghasabyan 10 , James Chandler 10 , Ivor S Douglas 11 , Eric P Schmidt 11 , Peter K Moore 9 , Franklin L Wright 2 , Ramona Ramdeo 4 , Robert Borrego 12 , Mario Rueda 12 , Achal Dhupa 5 , D Scott McCaul 5 , Tala Dandan 5 , Pralay K Sarkar 7 , Benazir Khan 7 , Coimbatore Sreevidya 7 , Conner McDaniel 8 , Heather M Grossman Verner 13 , Christopher Pearcy 8 , Lorenzo Anez-Bustillos 14 , Elias N Baedorf-Kassis 15 , Rashi Jhunjhunwala 14 , Shahzad Shaefi 16 , Krystal Capers 16 , Valerie Banner-Goodspeed 16 , Daniel S Talmor 16 , Angela Sauaia 17 , Michael B Yaffe 18
|
Background
Pulmonary vascular microthrombi are a proposed mechanism of COVID-19 respiratory failure. We hypothesized that early administration of tissue plasminogen activator (tPA) followed by therapeutic heparin would improve pulmonary function in these patients.
Research Question
Does tPA improve pulmonary function in severe COVID-19 respiratory failure, and is it safe?
Study Design and Methods
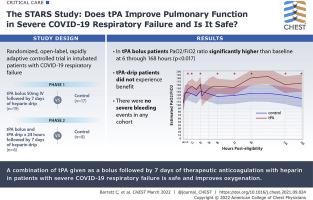
Adults with COVID-19-induced respiratory failure were randomized from May14, 2020 through March 3, 2021, in two phases. Phase 1 (n = 36) comprised a control group (standard-of-care treatment) vs a tPA bolus (50-mg tPA IV bolus followed by 7 days of heparin; goal activated partial thromboplastin time [aPTT], 60-80 s) group. Phase 2 (n = 14) comprised a control group vs a tPA drip (50-mg tPA IV bolus, followed by tPA drip 2 mg/h plus heparin 500 units/h over 24 h, then heparin to maintain aPTT of 60-80 s for 7 days) group. Patients were excluded from enrollment if they had not undergone a neurologic examination or cross-sectional brain imaging within the previous 4.5 h to rule out stroke and potential for hemorrhagic conversion. The primary outcome was Pao2 to Fio2 ratio improvement from baseline at 48 h after randomization. Secondary outcomes included Pao2 to Fio2 ratio improvement of > 50% or Pao2 to Fio2 ratio of ≥ 200 at 48 h (composite outcome), ventilator-free days (VFD), and mortality.
Results
Fifty patients were randomized: 17 in the control group and 19 in the tPA bolus group in phase 1 and eight in the control group and six in the tPA drip group in phase 2. No severe bleeding events occurred. In the tPA bolus group, the Pao2 to Fio2 ratio values were significantly (P < .017) higher than baseline at 6 through 168 h after randomization; the control group showed no significant improvements. Among patients receiving a tPA bolus, the percent change of Pao2 to Fio2 ratio at 48 h (16.9% control [interquartile range (IQR), –8.3% to 36.8%] vs 29.8% tPA bolus [IQR, 4.5%-88.7%]; P = .11), the composite outcome (11.8% vs 47.4%; P = .03), VFD (0.0 [IQR, 0.0-9.0] vs 12.0 [IQR, 0.0-19.0]; P = .11), and in-hospital mortality (41.2% vs 21.1%; P = .19) did not reach statistically significant differences when compared with those of control participants. The patients who received a tPA drip did not experience benefit.
Interpretation
The combination of tPA bolus plus heparin is safe in severe COVID-19 respiratory failure. A phase 3 study is warranted given the improvements in oxygenation and promising observations in VFD and mortality.
Trial Registry
ClinicalTrials.gov; No.: NCT04357730; URL: www.clinicaltrials.gov
中文翻译:

阿替普酶治疗 SARS-CoV-2 COVID-19 呼吸衰竭的研究
背景
肺血管微血栓是 COVID-19 呼吸衰竭的一种拟议机制。我们假设早期使用组织型纤溶酶原激活剂 (tPA),然后使用治疗性肝素会改善这些患者的肺功能。
研究问题
tPA 能否改善严重 COVID-19 呼吸衰竭患者的肺功能,是否安全?
研究设计和方法
从 2020 年 5 月 14 日到 2021 年 3 月 3 日,患有 COVID-19 引起的呼吸衰竭的成年人被随机分为两个阶段。第 1 阶段 (n = 36) 包括对照组(标准护理治疗)与 tPA 推注(50-mg tPA IV 推注,随后 7 天肝素;目标激活部分促凝血酶原激酶时间 [aPTT],60-80 秒) 团体。第 2 阶段 (n = 14) 包括对照组与 tPA 滴注(50-mg tPA IV 推注,随后 tPA 滴注 2mg/h 加肝素 500 单位/小时超过 24 小时,然后肝素维持 60-80 的 aPTT s 7 天)组。如果患者在过去 4.5 小时内未接受神经系统检查或横截面脑成像以排除中风和出血性转化的可能性,则将其排除在入组之外。主要结果是 Pa o 2至 F io 2随机化后 48 小时,比基线值有所改善。次要结局包括 Pa o 2与 F io 2比值改善 > 50% 或 Pa o 2与 F io 2比值在 48 小时时≥ 200(复合结局)、无呼吸机天数 (VFD) 和死亡率。
结果
50 名患者被随机分组:1 期对照组 17 名,tPA 推注组 19 名,对照组 8 名,2 期 tPA 滴注组 6 名。没有发生严重出血事件。在 tPA 推注组中,随机分组后 6 至 168 小时,Pa o 2与 F io 2比值显着(P < .017)高于基线;对照组无明显改善。在接受 tPA 推注的患者中,48 小时时 Pa o 2与 F io 2比率的百分比变化(16.9% 对照[四分位距 (IQR),–8.3% 至 36.8%] vs 29.8% tPA 推注 [IQR,4.5% -88.7%];P = .11),综合结果(11.8% vs 47.4%;P = .03)、VFD (0.0 [IQR, 0.0-9.0] vs 12.0 [IQR, 0.0-19.0]; P = .11) 和住院死亡率 (41.2% vs 21.1%; P = .19)与对照组相比,没有达到统计学上的显着差异。接受 tPA 滴注的患者没有获益。
解释
在严重的 COVID-19 呼吸衰竭中,tPA 丸加肝素的组合是安全的。鉴于氧合的改善以及 VFD 和死亡率的有希望的观察结果,有必要进行 3 期研究。
试验登记处
临床试验.gov;编号:NCT04357730;网址:www.clinicaltrials.gov


























 京公网安备 11010802027423号
京公网安备 11010802027423号